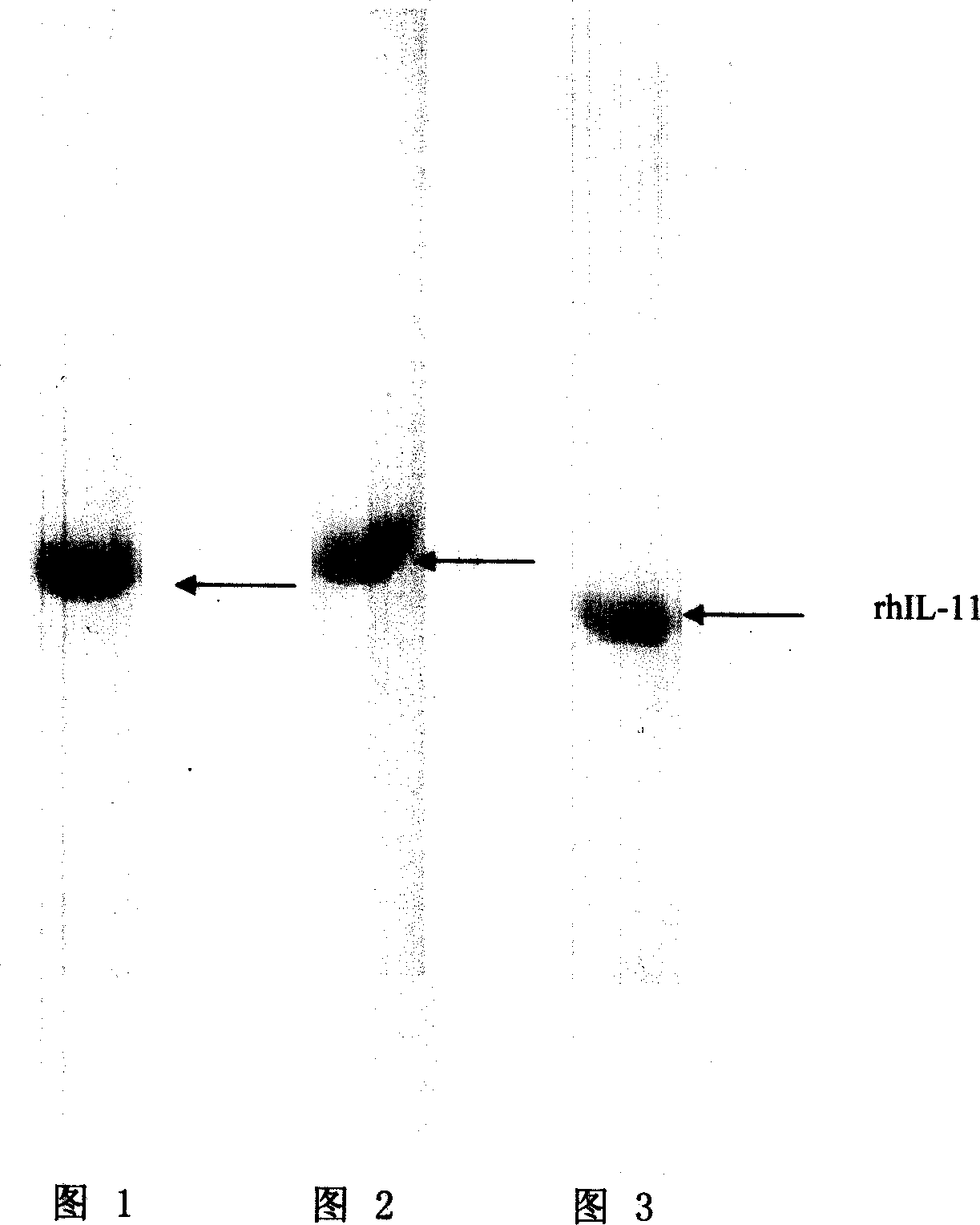High efficiency finished purification method for colibacillus expression recombinant human interleukin-11
A technology of human interleukin and Escherichia coli, which is applied in the field of medicine, can solve the problems of sample loading flow rate, low sample volume, target protein adsorption loss, and downstream purification difficulties, so as to improve purity and process stability, protein yield and quality Improve the effect of strong process stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] 1. Use Sephadex G-25 desalting column to desalt the fusion protein hydroxylamine cleavage solution, and collect the desalted solution after cutting.
[0037] 2. First connect the anion and cation exchange chromatography columns in series, the chromatographic medium of the anion exchange chromatographic column is agarose gel (DEAE-Sepharose F.F), the chromatographic medium of the cation exchange chromatographic column is agarose gel (CM -Sepharose F.F).
[0038] 3. Use pH 10.0, concentration 0.1mol / LGly-NaOH (glycine-sodium hydroxide) buffer solution to equilibrate the series columns, after equilibrating about 10 column volumes, load the desalted solution, and control the loading pressure below 0.3bar .
[0039] 4. After loading the sample, wash 10 column volumes with pH 10.0, concentration 0.1mol / L Gly-NaOH (glycine-sodium hydroxide) buffer solution. After washing, anion exchange chromatography column and cation exchange chromatography column Separate, wash the cation...
Embodiment 2
[0043] 1. Use Sephadex G-25 desalting column to desalt the hydroxylamine cleavage solution of the fusion protein, and collect the desalted solution after cutting.
[0044] 2. Use pH 9.2, concentration 0.3mol / LGly-NaOH (glycine-sodium hydroxide) buffer solution to equilibrate the anion-exchange chromatography column and the cation-exchange chromatography column respectively, and the chromatographic medium of the anion-exchange chromatography column is polymer Styrene 30Q (SOURCE 30Q), the chromatographic medium of the cation exchange chromatography column is polystyrene 30S (SOURCE 30S).
[0045] 3. Use pH 9.2, concentration 0.3mol / LGly-NaOH (glycine-sodium hydroxide) buffer solution to balance about ten column volumes respectively, and load the desalted solution in series on the anion exchange chromatography column and the cation exchange chromatography column , The sample pressure is controlled below 0.3bar.
[0046] 4. After loading the sample, wash 10 column volumes with t...
Embodiment 3
[0050] 1. Use Sephadex G-25 desalting column to desalt the hydroxylamine cleavage solution of the fusion protein, and collect the desalted solution after cutting.
[0051] 2. The anion and cation exchange chromatography columns are first connected in series, the chromatography medium of the anion exchange chromatography column is Sepharose (Q-Sepharose F.F), and the chromatography medium of the cation exchange chromatography column is Sepharose (SP-Sepharose F.F). Sepharose F.F).
[0052] 3. Use pH 7.0, concentration 0.2mol / L Gly-NaOH (glycine-sodium hydroxide) buffer solution to equilibrate the column in series, after equilibrating about 10 column volumes, load the desalted solution, and the pressure of the sample is controlled at 0.3bar the following.
[0053] 4. After loading the sample, wash 10 column volumes with the buffer described in step 3. After washing, separate the anion-exchange chromatography column from the cation-exchange chromatography column, and wash the ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com