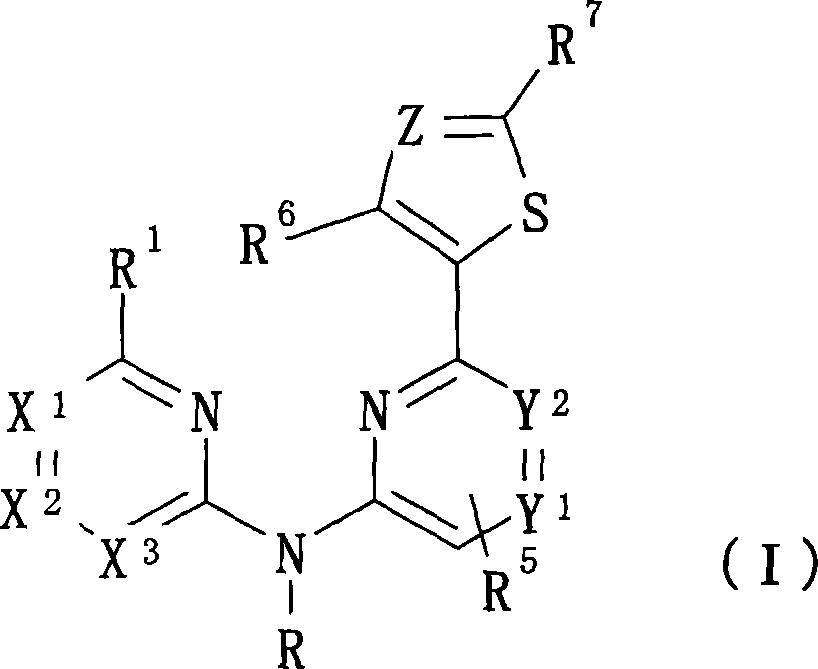
Novel aminopyridine compounds having Syk inhibitory activity
A technology of aminopyridine and compounds, which is applied in the field of therapeutic drugs for allergic diseases and aminopyridine compounds, and can solve the problems of low specificity and immunosuppression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[1887] Manufacturing Example 1: (Process Diagram 1)
[1888]
[1889] (X represents a leaving group such as a halogen atom, and R 7 ' means R 7 Nucleophilic substituents. The other symbols have the same meanings as described above. )
[1890] first process
[1891] Compound (2) is halogenated in a solvent such as dichloromethane, 1,2-dichloroethane, chloroform, carbon tetrachloride, acetonitrile, toluene, etc., using a halogenating agent such as thionyl chloride, oxalyl chloride, or in three In the presence of bases such as ethylamine, N,N-diisopropylethylamine, and pyridine, the reaction is carried out using a leaving group-introducing reagent such as methanesulfonyl chloride, p-toluenesulfonyl chloride, or trifluoromethanesulfonic anhydride to obtain compound (3).
[1892] second process
[1893] Compound (4) in ethanol, isopropanol, ethyl acetate, tetrahydrofuran, N,N-dimethylformamide, dimethyl sulfoxide, chloroform, acetonitrile, ethylene glycol dimethyl ether...
manufacture example 2
[1898] Manufacturing Example 2: (Process Diagram 2)
[1899] (The symbols in the figure have the same meanings as those described above.)
[1900]
[1901] Fifth process
[1902] By following the method described in Organic Letters, 5(18), 3233-3236, (2003), in solvents such as acetonitrile, tetrahydrofuran, 1,4-dioxane, N,N-dimethylformamide, magnesium chloride In the presence of bases such as triethylamine and N,N-diisopropylethylamine, nicotinic acid chloride compound (8) is reacted with malonic acid compound (9), and then concentrated hydrochloric acid is used for simultaneous decarboxylation and tert-butyl Deprotection of the oxycarbonyl group affords compound (10).
[1903] Sixth process
[1904] By making the compound ( 10) react with compound (11) to obtain compound (12). In this reaction, a base such as pyridine, triethylamine, N,N-diisopropylethylamine or the like may be used depending on the case. When the compound (11) is a carboxylic acid compound, ...
manufacture example 3
[1907] Manufacturing Example 3: (Process Diagram 3)
[1908]
[1909] Eighth process
[1910] Compound (15) can be obtained by reacting compound (7) and compound (14) according to the method shown in the fourth step.
[1911] Ninth process
[1912] In the presence of tetrakis (triphenylphosphine) palladium and sodium bicarbonate, potassium bicarbonate, sodium carbonate, potassium carbonate and other bases, in dimethoxyethane, diethyl ether, acetone, methyl ethyl ketone, Compound [I] can be obtained by reacting compound (15) with compound (16) in a solvent such as dioxane or tetrahydrofuran.
[1913] where the substituent R is required 7 In the case of a compound [I] having a "-CONH-bond", it can be obtained by combining a compound having a "-COOH group" and a compound having a "-NH 2 Compounds with the "group" can undergo amidation reaction to obtain the compound [I] with the desired "-CONH-bond".
[1914] In addition, where the substituent R is required 7 with "-N(-(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com