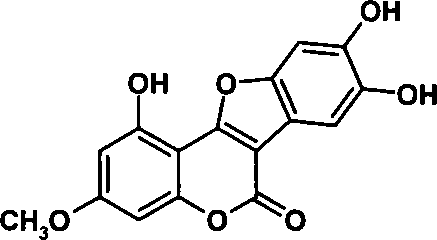
Chemical total synthesis method for wedelolactone
A technology for total synthesis of werethrin, which is applied in the field of medicine and chemical industry, can solve the problems of cumbersome operation process, high synthesis cost, and difficulty in obtaining, and achieves the effects of simplifying the operation process, reducing the cost, and reducing the synthesis cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
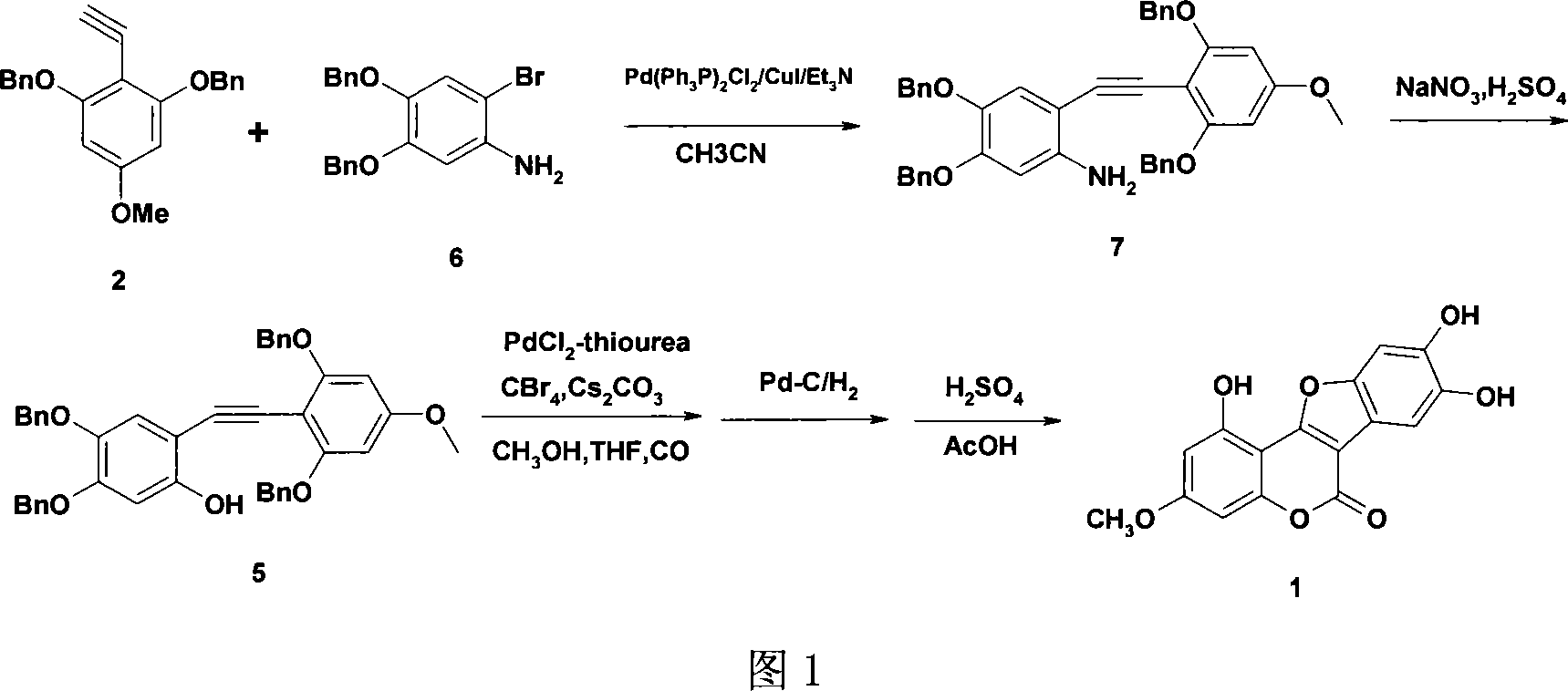
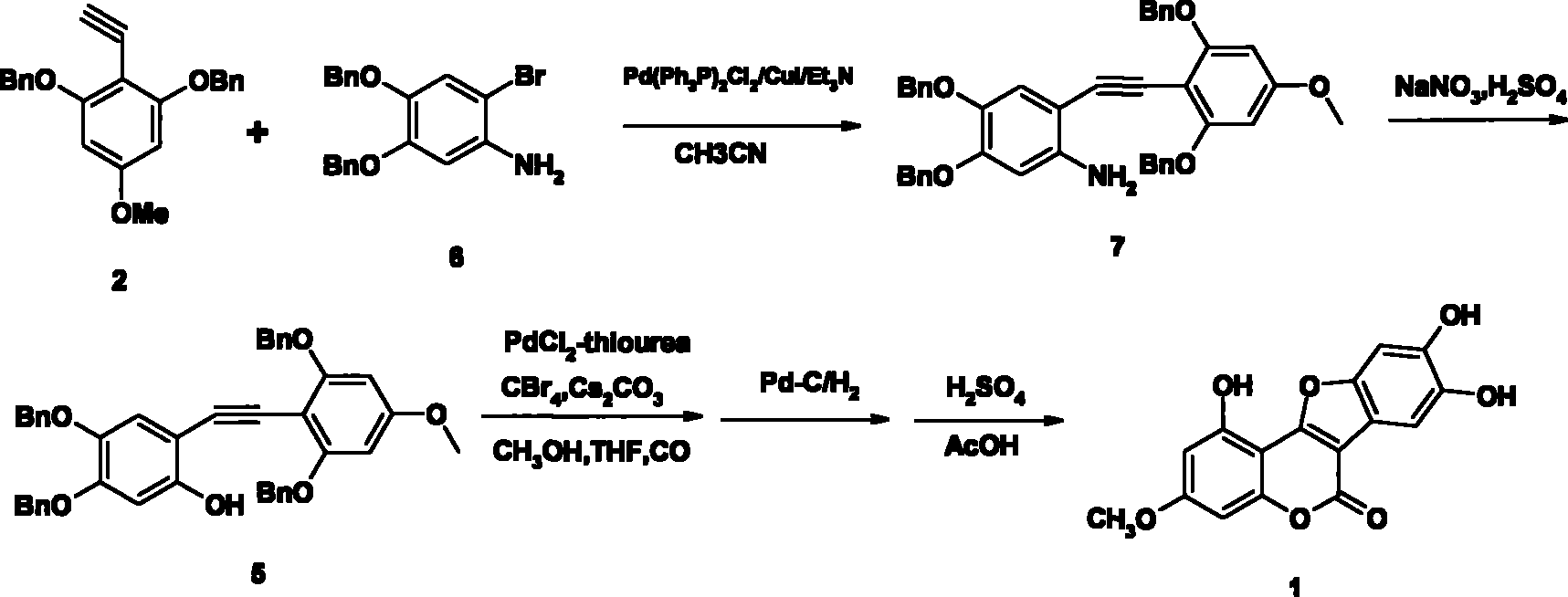
[0030] Preparation of 2-[(4-methoxy-2,6-dibenzyloxyphenyl)ethynyl]-4,5-dibenzyloxyaniline 7
[0031] Add 2-ethyne-5-methoxy-1,3-dibenzyloxybenzene 2 (7.4g, 21.45mmol), 2-bromo-4,5-dibenzyloxyaniline 6 (9.6 g, 25mmol) and acetonitrile (100ml), stirred for 1 hour under nitrogen protection, then added bistriphenylphosphine palladium dichloride (265mg, 0.38mmol), cuprous iodide (73mg, 0.38mmol) and triethyl Amine (18ml), reacted at room temperature under nitrogen protection for 18 hours, filtered, concentrated, extracted with ethyl acetate, washed the organic layer once with water and saturated brine, dried with magnesium sulfate, concentrated, and recrystallized with ethanol to obtain a solid , 2-[(4-methoxy-2,6-dibenzyloxyphenyl)ethynyl]-4,5-dibenzyloxyaniline 12.7g, yield 91.5%, palladium catalyst for recycling, melting point 191 -193°C.
Embodiment 2
[0033] Preparation of 2-[(4-methoxy-2,6-dibenzyloxyphenyl)ethynyl]-4,5-dibenzyloxyphenol 5
[0034] Add 2-[(4-methoxy-2,6-dibenzyloxyphenyl)ethynyl]-4,5-dibenzyloxyaniline (10.0g, 15.46mmol) and 20% sulfuric acid in a 250ml reaction flask (40mmol) was cooled to -5°C, and a solution of sodium nitrite (1.2g, 17.3mmol) and water (10ml) was added dropwise at -7°C to -5°C, and after 4 hours of reaction, the temperature was raised to 55°C to 60°C to react 1 hour, cooled, filtered, and recrystallized from isopropanol to give a white solid, 2-[(4-methoxy-2,6-dibenzyloxyphenyl)ethynyl]-4,5-dibenzyloxy Phenol 8.9g, yield 88.9%, melting point: 148°C-150°C.
Embodiment 3
[0036] Preparation of Methyl 2-[(4-methoxy-2,6-dibenzyloxyphenyl)-5,6-dibenzyloxy]-3-benzofurancarboxylate
[0037] Add 2-[(4-methoxy-2,6-dibenzyloxyphenyl)ethynyl]-4,5-dibenzyloxyphenol (6.5g, 10.0mmol) in a 500ml autoclave, iodine Cuprous chloride (191mg, 1.0mmol), urea (75mg, 1.0mmol), palladium dichloride (175mg, 1.0mmol), carbon tetrabromide (25g, 75.3mmol), cesium carbonate (25.5g, 75.3mmol) , methyl alcohol (160ml), tetrahydrofuran (80ml), feed into carbon monoxide for ventilation, under 5 atmospheres, react 5 hours under 80 ℃, cooling, after the gas in the still with nitrogen replacement, stop reaction with ammonium chloride aqueous solution (15ml), The catalyst was recovered, the reaction solution was extracted with ethyl acetate, the organic layer was washed once with saturated aqueous ammonium chloride and saturated brine, dried with magnesium sulfate, concentrated, and recrystallized with ethanol to obtain solid 2-[(4 -Methoxy-2,6-dibenzyloxyphenyl)-5,6-dibenzylox...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com