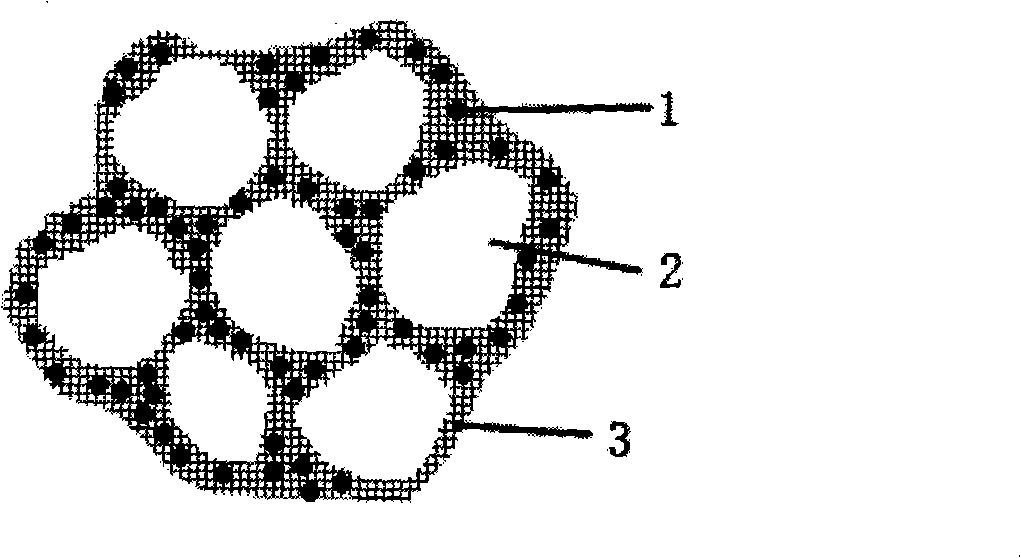
Novel orthopaedics medicaments carrier system and preparation thereof
A carrier system and drug technology, applied in drug delivery, drug combination, pharmaceutical formulation, etc., can solve the problems of slow degradation, no osteoconductivity and osteoinductivity, and incomplete degradation, and achieve high reactivity and good bioavailability Degradability, effects of good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Bioactive glass-based vancomycin drug carrier
[0039] Use analytical pure Na 2 CO 3 , CaCO 3 , H 3 BO 3 , Na 2 HPO 4 As raw materials, prepare batch materials according to the glass composition in Table 1. After mixing the batch materials uniformly, melt them in a platinum crucible at 1100~1150°C for 2 hours to obtain bubble-free glass liquid, and finally quench the molten glass , To obtain a transparent crystal-free glass block. Crush the block, grind and sieve the glass powder between 25 and 50 μm.
[0040] Table 1 The chemical composition of bioactive glass
[0041]
[0042] In addition, 40 mg of vancomycin was dissolved in 0.2 ml of phosphoric acid curing buffer, stirred well, and 1 g of sieved glass powder was added, and the mixture was stirred evenly and then filled into the mold. Press 10MPa for 15s, after demolding, put it into the environment with humidity>95% and temperature at 37℃ for 5h to obtain the drug carrier. The strength of the drug carrier is...
Embodiment 2
[0045] Example 2 Bioactive glass-based rifampicin drug carrier
[0046] Use analytical pure Na2CO3, CaCO3, H3BO3, SiO2, Na 2 HPO 4 As raw materials, batch materials were prepared according to the glass composition shown in Table 2. After mixing the batch materials uniformly, melting them in a platinum crucible at 1200-1250°C for 2 hours to obtain a bubble-free glass solution, and finally quenching the molten glass solution to obtain a transparent crystal-free glass. Grind and sieve glass powder between 25 and 50 μm. Mix 40 mg of rifampicin with 700 mg of glass powder thoroughly, drop in 0.2 ml of phosphoric acid curing buffer, stir well and fill it into the mold. No pressure is applied, free forming. Put the mold into an environment with a humidity of >95% and a temperature of 37° C. for aseptic curing for 5 hours, and demold the mold to obtain a drug carrier. The drug strength is 15-20MPa.
[0047] Table 2 Chemical composition of bioactive glass
[0048]
[0049] Dry the drug c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com