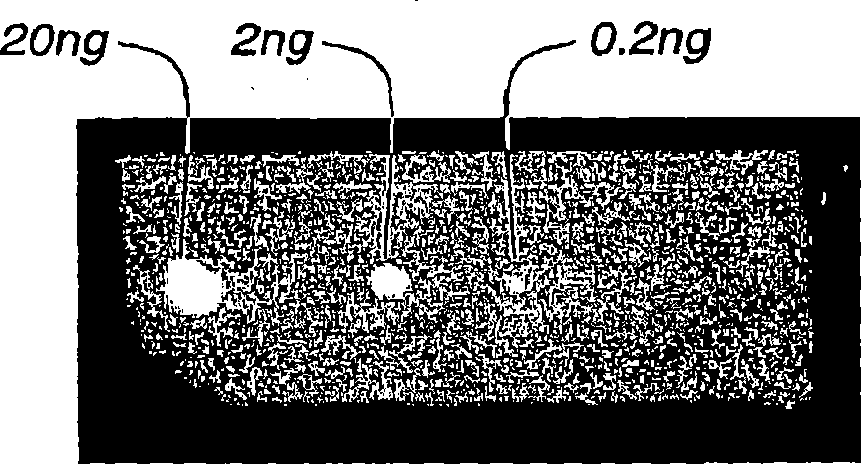
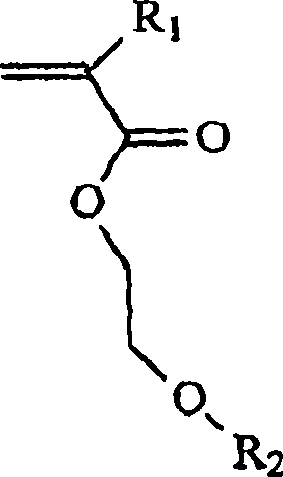
Loaded latex optical molecular imaging probes
A technology of latex particles and latex materials, which is applied in the field of fluorescent probes for optical molecular images, can solve problems such as easy bleaching, non-emission, and colloidal instability, and achieve the effects of enhancing fluorescence effects, resisting adhesion, and good dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0238] Dye Synthesis Example 1 - Preparation of Dye 1
[0239] Using 2,3,3-trimethyl-1-octadecyl-3H-indolium perchlorate (4.28g, 10mmol) and dianiline (1.4g, 5mmol) in 40ml containing triethylamine ( 1.5 g, 15 mmoles) of acetic anhydride, the dye was prepared according to the general procedure above. The reaction time was 5 minutes. The reaction was cooled to 25°C and poured into 2 liters of ice water with vigorous stirring. The water layer was separated, and the oil layer was dissolved in 100 mL of 80 / 20 dichloromethane-methanol solution. The material was chromatographed on a silica gel column eluting with 80 / 20 dichloromethane-methanol solution. After drying over anhydrous magnesium sulfate the solution was evaporated to give pure dye (4 g, 32% yield). λmax in methanol = 747 nm, extinction coefficient = 220,020.
[0240] Dye Synthesis Example 2-Preparation of Dye 5
[0241] Using 2,3,3-trimethyl-1-butyl-3H-indolium perchlorate (12g, 38mmol) and dianiline (5.4g, 19mmol)...
Embodiment 10
[0260] Embodiment 10. Preparation of amine-terminated polyethylene glycol macromonomer
[0261]
[0262] Poly(ethylene glycol dimethacrylate) (Aldrich, Mn=875, 335 g) was mixed with 100 mL of methanol and treated with cysteamine (Aldrich, 5.8 g) and diisopropylethylamine (Hunigs base), then at room temperature After stirring for 2 days, it was concentrated on a rotary evaporator. The resulting residue was dissolved in 1 L of ethyl acetate and extracted with 10% aqueous hydrochloric acid. The aqueous layer was collected and made alkaline by adding 50% aqueous sodium hydroxide solution, followed by extraction with ethyl acetate. The organic layer was dried over magnesium sulfate and concentrated. The resulting residue was dissolved in anhydrous diethyl ether and treated with gaseous hydrogen chloride and stood. The ether was decanted leaving a dark blue oil. It was rinsed with fresh diethyl ether and the diethyl ether was poured off. The dark blue oil was concentrated on...
Embodiment 11
[0264] Example 11. Contains methoxyethyl methacrylate (45% w / w), divinylbenzene (4%), ethylstyrene (1%) and polyethylene glycol monomethyl ether methyl Preparation of Nanolatex 1 of Acrylate (50%)
[0265] A 500ml three-neck flask was bottomed with an Ace #15 glass line and a series of adapters to allow it to be connected to 1 / 16 inch size teflon tubing. The flask (hereinafter referred to as the "header" flask) was equipped with a mechanical stirrer, a rubber septum with a needle syringe nitrogen inlet. In the Header flask, methoxyethyl methacrylate (5.63 g), divinylbenzene (0.63 g, containing a mixture of isomers with a purity of 80% of ethyl styrene isomers), poly Ethylene glycol monomethyl ether methacrylate (6.25g, Mn=1100), cetylpyridinium chloride (0.31g), 2,2'-azobis(N,N'-dimethylene isobutylamidine) dihydrochloride (0.06g), sodium bicarbonate (0.06g) and distilled water (159.13g). Into a 1 L three-neck round bottom flask equipped with a mechanical stirrer, reflux co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com