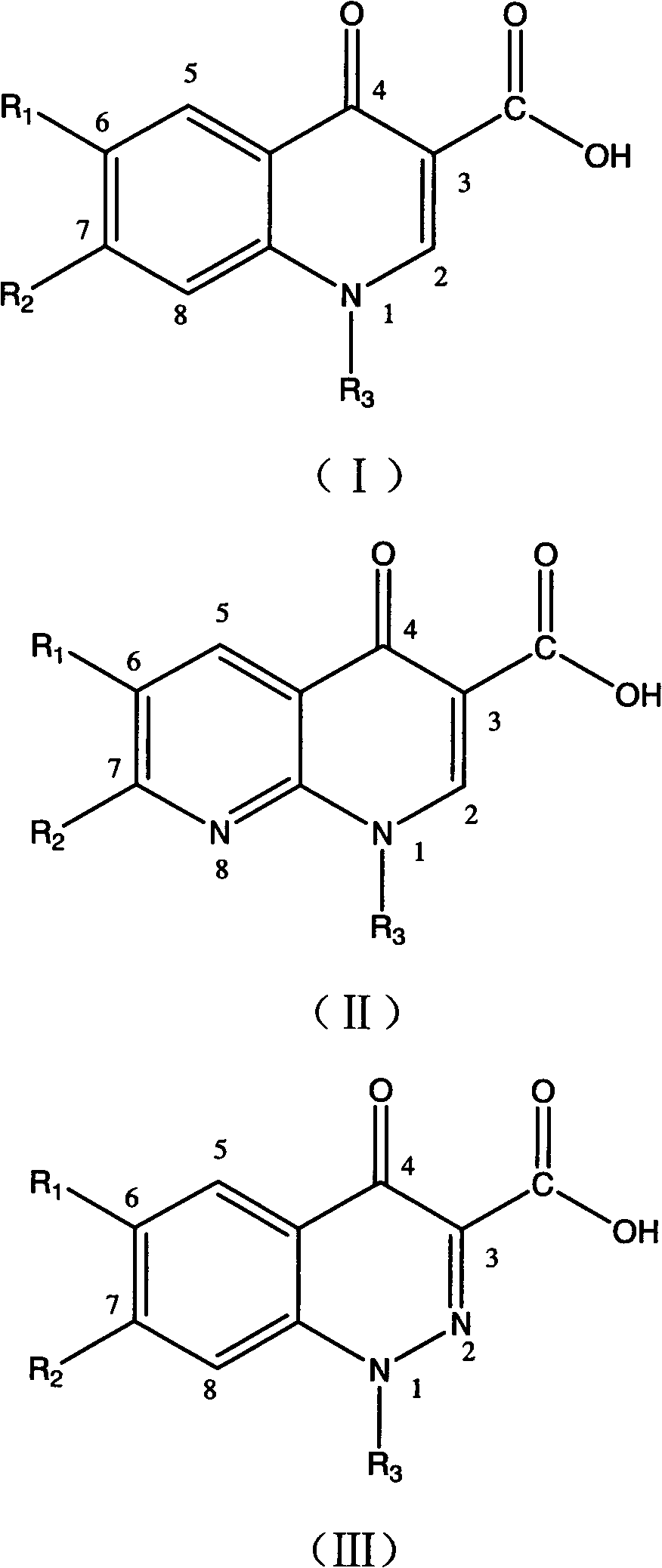
Detection method of quinolone drugs, detection reagent kit and application
A detection method, the technology of quinolones, is applied in the preparation of test samples, analysis by making materials undergo chemical reactions, and material analysis by observing the influence of chemical indicators, which can solve the problems that are not suitable for on-site detection and promotion, Complicated operation, poor specificity, etc., to achieve high specificity, simple operation, and low reagent cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1 specificity test
[0047] This embodiment selects metronidazole reference substance, chloramphenicol standard substance, cefpodoxime axetil reference substance, dexamethasone tablet, salicylic acid reference substance, berberine hydrochloride tablet, ofloxacin reference substance, gatiza Star reference substance, norfloxacin reference substance, ciprofloxacin reference substance and pefloxacin reference substance are used as the samples to be tested, and these eleven samples are tested. The detection methods are divided into the following two types:
[0048] method one:
[0049] (1) The samples to be tested are respectively prepared into solutions with a concentration greater than 1 mg / ml;
[0050] (2) configure 100mg / ml ferric ammonium sulfate solution, which contains 20% sulfuric acid;
[0051] (3) Add a drop of ferric ammonium sulfate solution prepared in step (2) to each sample solution to be tested prepared in step (1), and observe the color developm...
Embodiment 2
[0073] Embodiment 2 minimum detection limit test
[0074] This embodiment selects ofloxacin, lomefloxacin, enoxacin, ciprofloxacin, norfloxacin, levofloxacin, fleroxacin, pefloxacin, pazufloxacin, gatifloxacin, toluenesulfonate Tofloxacin acid and enrofloxacin hydrochloride are used as the samples to be tested, and the serial concentration solutions of these twelve samples are prepared respectively earlier, and the concentrations are: 0.04, 0.09, 0.15, 0.20, 0.25, 0.30, 0.38, 0.76, 1.52 and 3.00mg / ml;
[0075] According to the operation steps of Method 1 in Example 1, one drop of ferric ammonium sulfate solution was added to the serial concentration solutions of each sample to be tested, and the color development results were observed.
[0076] It can be seen from the color development results that the minimum detection limits of the twelve samples to be tested are all 0.20 mg / ml (visible to the naked eye), and the color development results are light orange red to orange red....
Embodiment 3
[0077] Example 3 Detection of Acne Eliminating Essence
[0078] In this example, the anti-acne Wuhen essence in the commercially available anti-acne Wuhen kit is selected as the sample to be tested, and whether it is illegally added with quinolones is tested.
[0079] Samples to be tested: acne-free essence, water, 20ml;
[0080] Chromogen: 0.1mg / ml ferric ammonium sulfate solution (without sulfuric acid);
[0081] Preprocessing:
[0082] (1) Remove 2ml of Acne Wuhen Essence, add 1ml of dichloromethane with a dropper, shake vigorously for 30 seconds, let stand, and collect the lower liquid;
[0083] (2) Add 1ml of 0.05mol / L hydrochloric acid aqueous solution to the above-mentioned lower layer liquid, shake vigorously for 30 seconds, let stand, and collect the upper layer liquid;
[0084] Detection: Add 1 drop of 0.1 mg / ml ferric ammonium sulfate solution to the above upper layer liquid, and it will be orange-red, which is judged as positive, indicating that the Acne Elimina...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com