Method for preparing human serum amyloid A1 and expression vector and genetic engineering bacteria thereof
A technology of genetically engineered bacteria and expression vectors, applied in the field of preparation of human serum amyloid A1 and its expression vectors and genetically engineered bacteria, can solve the problems of complicated purification methods, long time-consuming, low yield, etc., and achieve simplification of purification methods , avoid denaturation, low purification cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
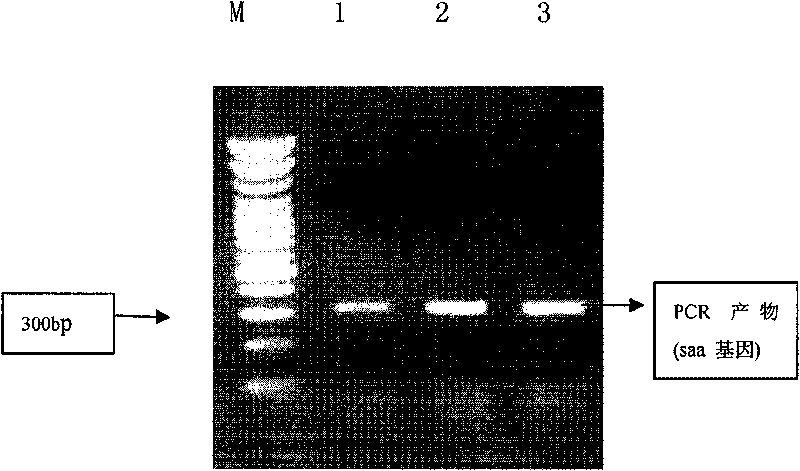
[0034] Example 1. PCR amplification of the coding gene of human SAA1 and construction of recombinant expression plasmid pET28a(+)-SAA
[0035] The build steps are as follows:
[0036] 1) Extract total RNA:
[0037] Fresh patient liver tissue was taken, crushed with a homogenizer, and total RNA was extracted with an RNA extraction kit.
[0038] 2) PCR amplification of the target gene (RT-PCR):
[0039] Using the total RNA as a template, the cDNA of SAA1 was obtained by using a reverse transcription kit. Design a pair of primers according to the human SAA1 sequence published in the gene bank, the primers are:
[0040] Upstream primer: 5'AT CATATG TTCTTTTCGTTCCTTGGCGAG(Nde I);
[0041] Downstream primer: 5'AT CTCGAG TCAGTATTTCTCAGGCAGG (Xhol I).
[0042] The primers were designed with reference to the reported sequence of human SAA1, starting from the 60th nucleotide of cDNA to amplify the mature peptide gene of human SAA protein.
[0043] The following PCR reaction sys...
Embodiment 2
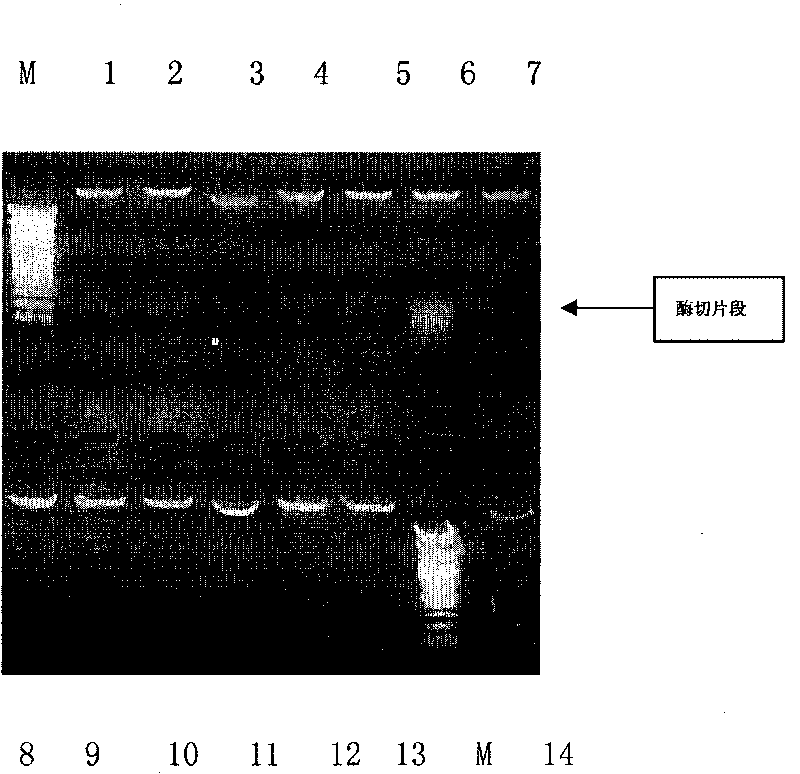
[0049] Example 2. Construction and screening of high-efficiency expression engineering bacteria
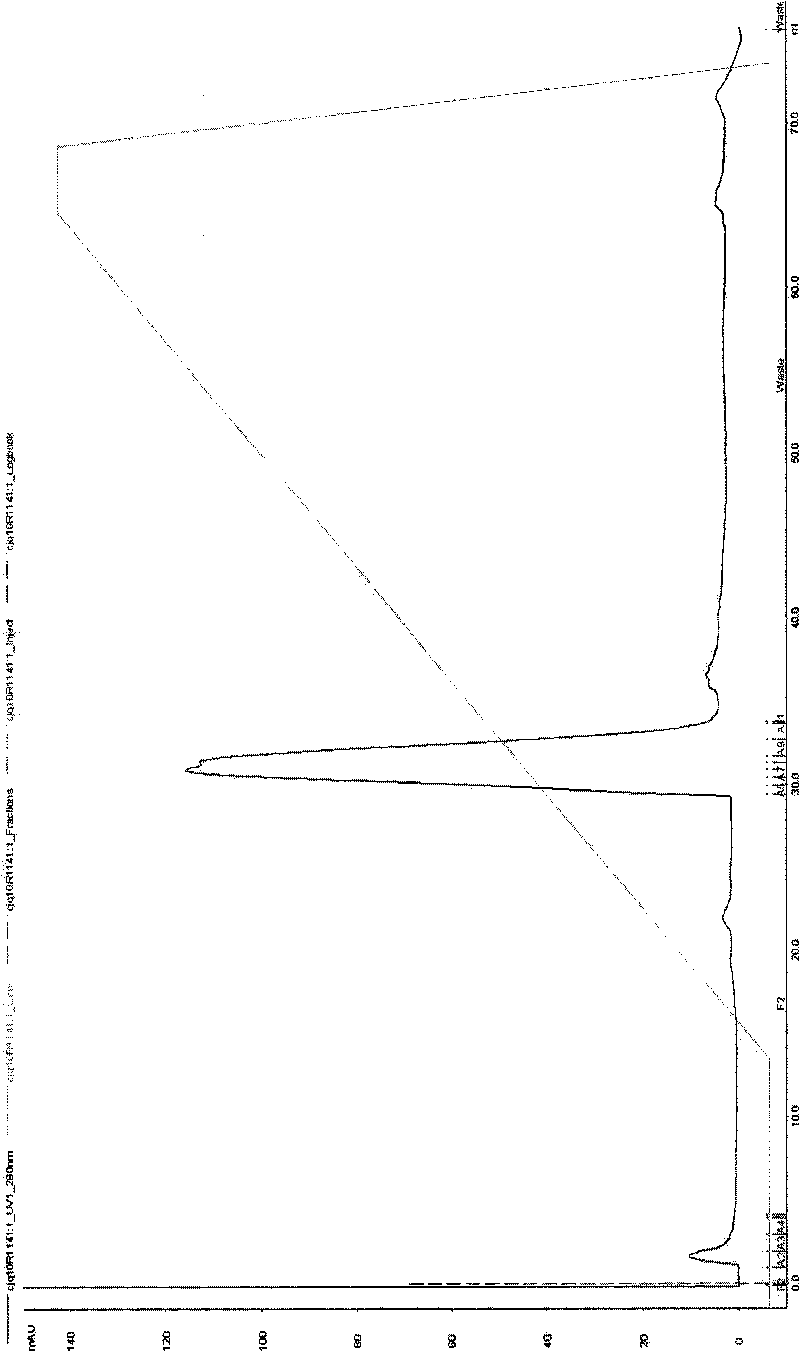
[0050] The recombinant plasmid pET-28(a)-SAA was transformed into Escherichia coli Rosetta-gami, and a single colony of the recombinant bacteria was inoculated in 3 ml of LB medium containing kanamycin, and cultured on a shaker at 37°C overnight. The next day, inoculate 2% of the overnight cultured recombinant engineered bacteria into 3ml LB medium, culture on a shaker at 37°C for about 2 hours until the OD600 is about 0.8, add IPTG to a final concentration of 1mM, and induce for 2.5 hours. 15% SDS-PAGE was used to detect the expression form and amount of the fusion protein, and to screen highly expressing strains. The results are shown in Table 2 below, with Figure 4a And attached Figure 4b . attached Figure 4a and 4b It shows that the results of the protein electrophoresis graph and the growth curve of the host bacteria show that after adding the inducer IPTG for a total...
Embodiment 3
[0055] Embodiment 3. Large-scale expression and purification method of gene recombinant bacteria
[0056] Expression of recombinant human SAA-polyhistidine fusion protein in Escherichia coli host ;
[0057] 1) Transform PET28a-saa1 into Rosetta-gami Escherichia coli on an LB culture plate with kanamycin resistance (50ng / ml), culture overnight at 37°C;
[0058] 2) Pick a single colony and culture it overnight at 37°C at 250 rpm in 50ml of LB culture containing 50μg / ml kanamycin;
[0059] 3) 2% inoculated into 1L LB medium (including 50ug / ml kanamycin), 37°C to A600, O.D.0.5;
[0060] 4) Add IPTG to a final concentration of 1mM at 37°C and induce culture at 300 rpm for 3 hours;
[0061] 5) Centrifuge at 4000 rpm for 20 minutes at high speed, collect the precipitate and store it at -80°C.
[0062] Reagent for Purifying Recombinant Human SAA-Polyhistidine Fusion Protein
[0063] 1) Lysis solution: 50mM Tris-HCl Ph 8.0, 500mM NaCl, 10mM imidazole, 1mMPMSF, 5mM 2-Me
[0064...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com