Quinacridone derivative borate and preparation method and application thereof
A kind of compound and sulfonic acid group technology, applied in the field of quinacridone derivative boronate ester and preparation thereof, can solve the problems of difficult tin alkylation or boronate esterification, not much, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
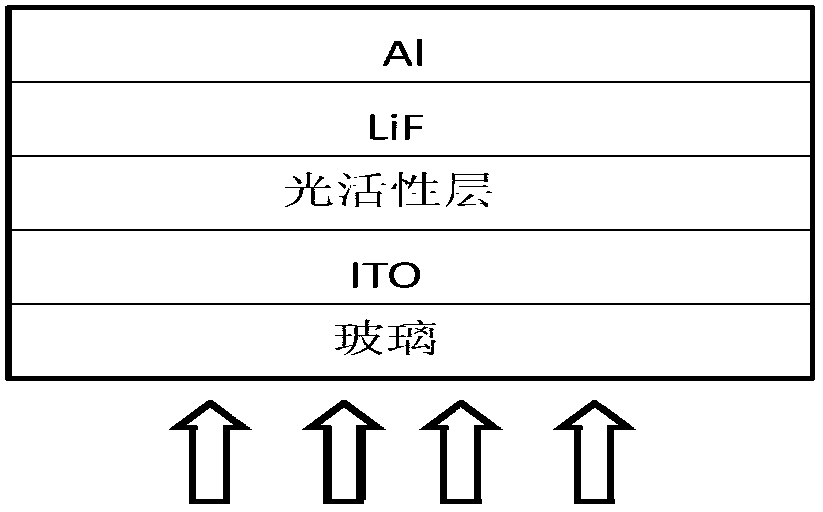
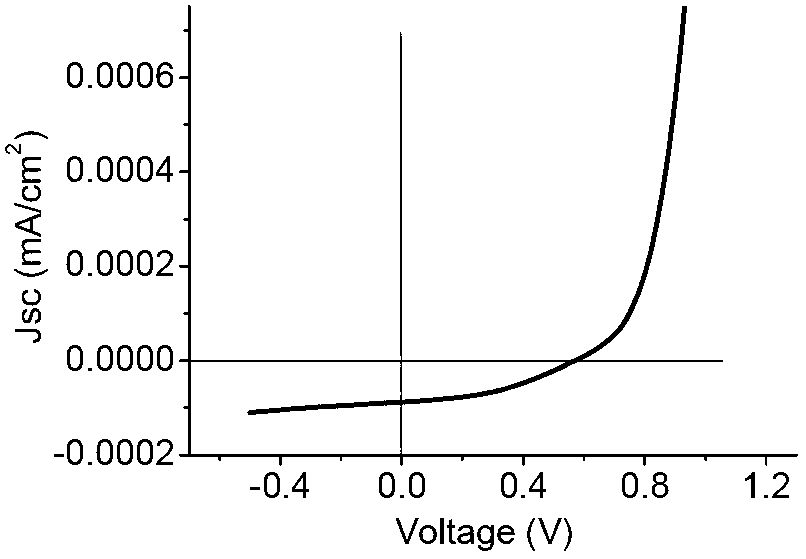
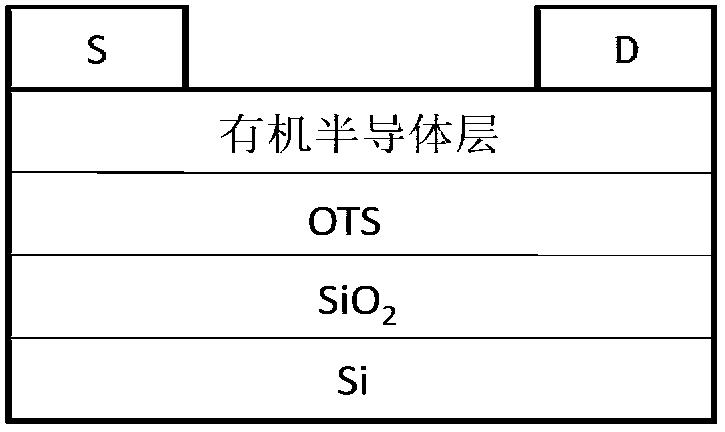
Image
Examples
Embodiment 1
[0086] Embodiment 1: the synthesis (R 1 Synthesis of 2-octyl-dodecyl) quinacridone derivative boronate
[0087]Synthesize compound C-1 first, the structural formula is as follows:
[0088]
[0089] Compounds A-1 and B-1 of the following structures are involved in the synthesis process:
[0090]
[0091] First, in a system containing a strong base, a phase transfer catalyst, and toluene, quinacridone and R-Br react to obtain A-1. Compound A-1 reacts with liquid bromine under weakly acidic conditions to obtain B-1. For the specific preparation process of A-1 and B-1, please refer to the literature: Applied materials & interfaces, 2010, 2, 2679.
[0092] In a 250 ml two-necked flask, add 500 mg of B-1 to 80 ml of acetic anhydride, add three times the molar equivalent of malononitrile, heat to reflux overnight, evaporate the acetic anhydride, and the solid is subjected to silica gel column chromatography, petroleum ether: Elution with ethyl acetate=2:1 gave C-1 in 25% yi...
Embodiment 2
[0097] Embodiment 2: the synthesis (R 1 for decanyl)
[0098] Synthesize compounds A-2 and B-2 first, and the structural formula is as follows:
[0099]
[0100] First, add 1 gram of quinacridone, 2.1 grams of tetrabutylammonium bromide, and 0.8 grams of sodium hydride into a 500 ml two-necked bottle, use toluene as a solvent, protect the temperature with argon, raise the temperature to 120 ° C for 2 hours, and drop 1 -Decane bromide, cooled after reacting overnight, spin-dried toluene, and the solid was subjected to silica gel column chromatography, eluting with petroleum ether: dichloromethane = 2:1 to obtain light green powder A-2 with a yield of 30%. Molecular ion peak: 592.6.
[0101] In a 250 ml two-necked bottle, add 400 mg of A-2, 0.8 mg of potassium acetate, and 100 ml of acetic acid, heat to 80°C, and inject 0.05 ml of Br 2 After reacting for 4 hours, it was extracted with chloroform and chromatographed on a silica gel column, eluting with petroleum ether: ethy...
Embodiment 3
[0108] Embodiment 3: compound shown in preparation formula II (R 1 is 2-octyl-dodecyl, Ar is preferably Ar-1)
[0109]
[0110] The preparation of compounds D, E, and F is involved in the process of preparing Ar-1, and the structural formula is as follows:
[0111]
[0112] Preparation of compounds D, E, F:
[0113] Under strong basic conditions, cyanothiophene and dimethyl succinate react to obtain compound D;
[0114] Compound D reacts with 1-bromo-2-octyl-dodecane in DMF solution containing weak base and phase transfer catalyst to obtain E. In chloroform, add compound E and N-bromosuccinimide to obtain F; reference for specific preparation process: Macromolecules, 2010, 43, 821.
[0115] The reaction equation of the compound shown in the preparation formula II with quinacridone derivative borate is as follows:
[0116]
[0117] The specific preparation process is: under the protection of argon, add 54 mg of compound quinacridone derivative borate, 90 mg of comp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com