Method for preparing strobilurin fungicide
A technology of methoxyacrylic acid and bactericide, which is applied in oxime preparation, organic chemistry, etc., can solve the problems of poor repeatability, difficult preparation, and high difficulty, and achieve a process environment-friendly, easy-to-obtain raw materials, and easy operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088]
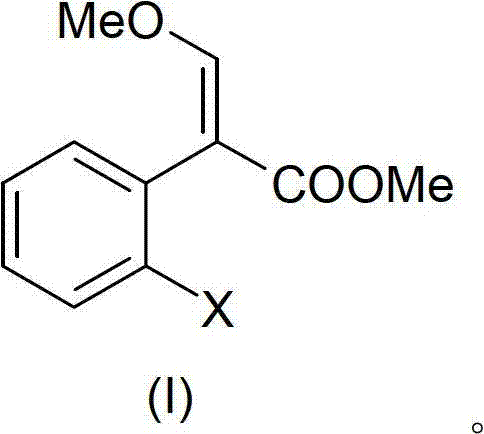
[0089]36.1 grams (0.1mol) (I) was dissolved in 100 milliliters of toluene, 15 grams (0.126mol) DMF-DMA was added, and a distillation device with a small rectification column was installed, and the temperature was slowly raised to 90 ° C, and low boiling fractions slowly Come out; control the distillation rate to be moderate, continue to heat up to 120 ° C, TLC tracking until the conversion of the raw material is complete. Evaporate under reduced pressure to remove the residual low boiler as the intermediate of azoxystrobin, cool and set aside.
Embodiment 2
[0091]
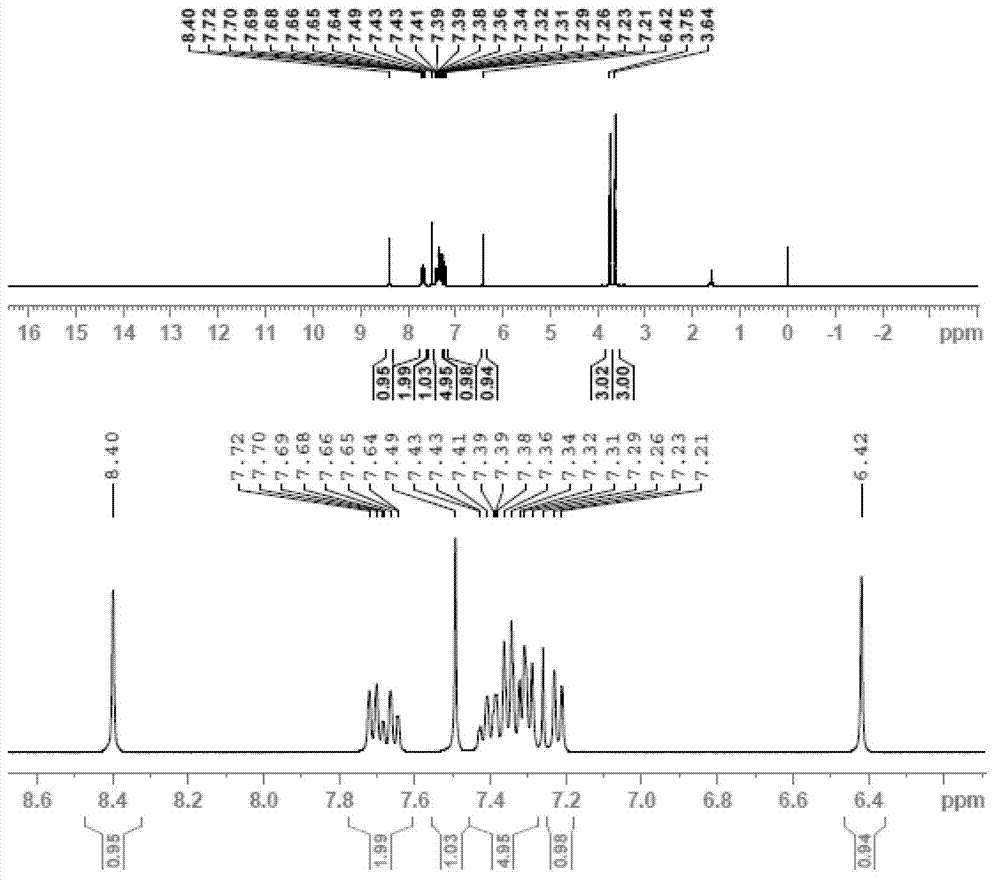
[0092] The product in embodiment (1) was dissolved in 50 milliliters of 10% hydrochloric acid, stirred at room temperature for 3 hours; extracted three times with 150 milliliters of toluene, combined the toluene phase, added 1 gram of benzyltriethylammonium chloride, 30 grams of 20 % sodium hydroxide solution and 20 grams of dimethyl sulfate, stirred at room temperature for 3 hours, and heated to 50 ° C for 2 hours; the toluene phase was separated, washed once with 20 ml of 5% hydrochloric acid, the toluene was concentrated under reduced pressure, and the residue was used in 20 ml Methanol recrystallization obtained 35 grams of azoxystrobin product, with a content of 98.5% (detected by external standard method), and a yield of 85.1%. 1 HNMR (CDCl 3 ), δ3.64 (s, 3H), 3.75 (s, 3H), 6.42 (s, 1H), 7.22 (d, 1H), 7.26-7.43 (m, 5H), 7.49 (s, 1H), 7.66 ( t, 1H), 7.71 (d, 1H), 8.4 (s, 1H)
Embodiment 3
[0094]
[0095] The product in embodiment (1) was dissolved in 80 milliliters of 20% hydrogen chloride / methanol solution, stirred at room temperature for 12 hours; Atmospheric distillation reclaimed most of the excess hydrogen chloride / methanol solution, then steamed under reduced pressure to remove low boilers, residue Add 1 gram of potassium bisulfate, heat to 140-150°C for 6 hours under reduced pressure with a water pump; cool to room temperature, add 150 ml of toluene, filter, wash once with 25 ml of 5% sodium bicarbonate, concentrate the toluene under reduced pressure, and the residue 32.9 g of the product was obtained by recrystallization with 20 ml of methanol, with a content of 98.8% (HPLC detected by external standard method), and a yield of 80.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com