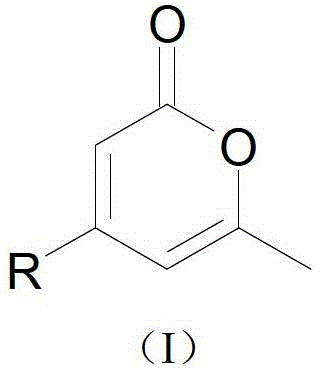
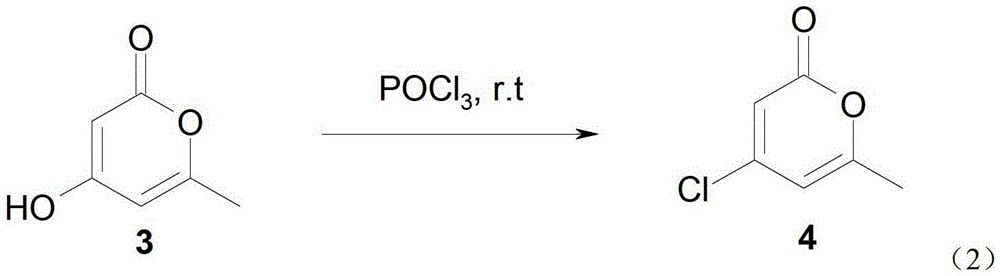4-substituted alpha-pyrone derivative as well as preparation method and application thereof
A pyrone and methyl technology, which is applied in the field of 4-substituted alpha-pyranone derivatives and their preparation, can solve the problem that 6-pentyl-dihydropyran-2-one cannot be stably synthesized and inoculants cannot be Stable performance of disease control activity, less types of pyrone derivatives, etc., to achieve the effects of easy industrial production, low cost, and high bacteriostatic rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
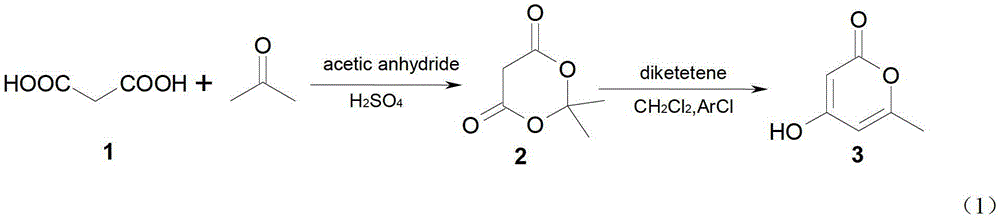
[0044] Example 1 Synthesis of 6,6-dimethyldihydro-2H-pyran-2,4(3H)-dione (Formula 2)
[0045] Suspend 52 grams of maleic acid powder in 60 milliliters of acetic anhydride, cool the system with water, add 1.5 milliliters of concentrated sulfuric acid while stirring, slowly add 40 milliliters of acetone to the above solution, and keep the temperature at 20-25 degrees (cooling is required). After reacting for 2 hours, the reaction bottle was placed in a refrigerator at 3-5°C overnight, filtered with suction, the crystals were fully washed with ice water three times, and dried to obtain 35 g of crude compound 1. The compound was dissolved in 70 ml of acetone, and an appropriate amount of water (about 140 ml) was added for crystallization to obtain 25 g of pure 6,6-dimethyldihydro-2H-pyran-2,4(3H)-dione, mp. 95-96°C. The structural formula is as follows:
[0046]
Embodiment 24
[0047] Synthesis of embodiment 24-hydroxyl-6-methyl-2-pyrone (formula 3)
[0048] 14.4 g (0.1 mol) of 6,6-dimethyldihydro-2H-pyran-2,4(3H)-dione prepared by the method in Example 1, 35 milliliters of dichloromethane and 15 milliliters of chlorobenzene Place in a reaction bottle, cool to below 5°C under stirring, add 10.1g (0.1mol) triethylamine and 10.1g (0.12mol) diketene in turn, slowly warm up to room temperature after addition, and continue stirring for 2 hours. The reaction solution was washed with 5% hydrochloric acid at 3-8°C, dried over anhydrous sodium sulfate and filtered to remove most of the dichloromethane. The remaining solution was heated to reflux for 2 hours, and yellow crystals precipitated out. Cool to crystallize, filter with suction and wash the crystals with a small amount of ether, and dry to give 10.1 g of 4-hydroxy-6-methyl-2-pyrone, yield 82%, mp.188~189°C. 1H NMR (CDCl 3 ) chemical shift: 2.245 (s, 3H, CH 3 ),5.484(d,1H,CH),5.885(s,1H,CH),1.557(s...
Embodiment 3
[0050] Example 3 Synthesis of 4-methoxyl-6-methyl-2-pyrone (5a)
[0051] Add 4-hydroxy-6-methyl-2-pyrone 3 (0.8g, 0.0063mol) solid, 0.55ml of bromomethane (0.0095mol) solution and 2ml of triethylamine (0.019mol) solution to a 50ml round bottom Add about 20ml of acetonitrile solution into the flask, stir to dissolve, and react in an oil bath at 40°C for 2 hours. Most of the solvent was removed by a rotary evaporator, and the product 4-methoxy-6-methyl-2-pyrone was obtained by direct column chromatography separation. The mobile phase ratio is about E:P=1:5, and it is a single fluorescent point under the ultraviolet lamp, with a small amount of raw material remaining at the origin (basically no fluorescence). Remove the treatment solvent, the product is a solid, the yield is about 50-70%, mp.88-89 ° C. H-NMR (300MHz, CDCl 3 ):2.205 (s,3H,CH 3 ), 3.790 (s,3H,CH 3 ),
[0052] 5.407(d,1H,CH),5.774(q,1H,CH).
[0053] HR-MS (ESI) m / z: 141.0553 (M+H + ,100%). The structural for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com