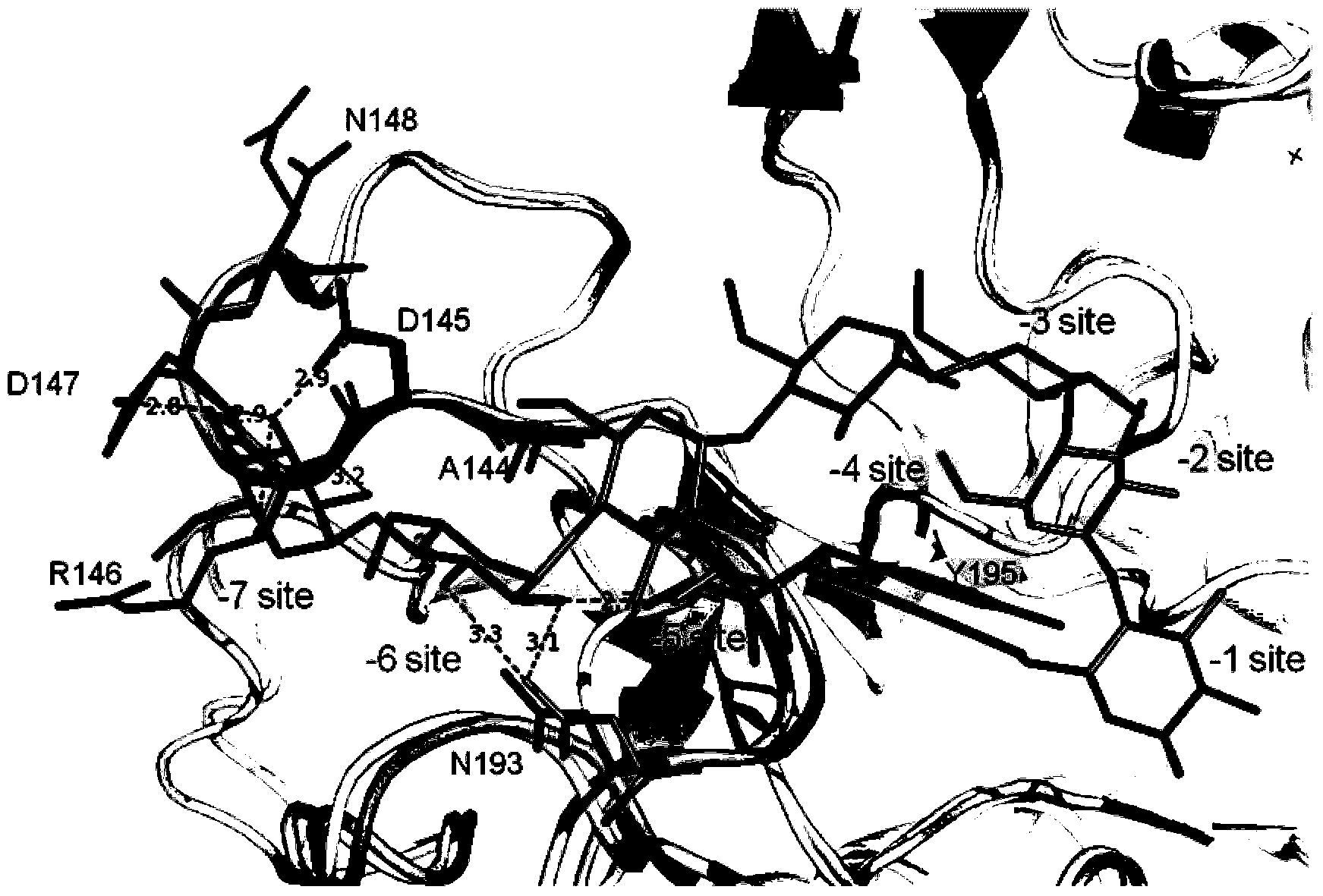
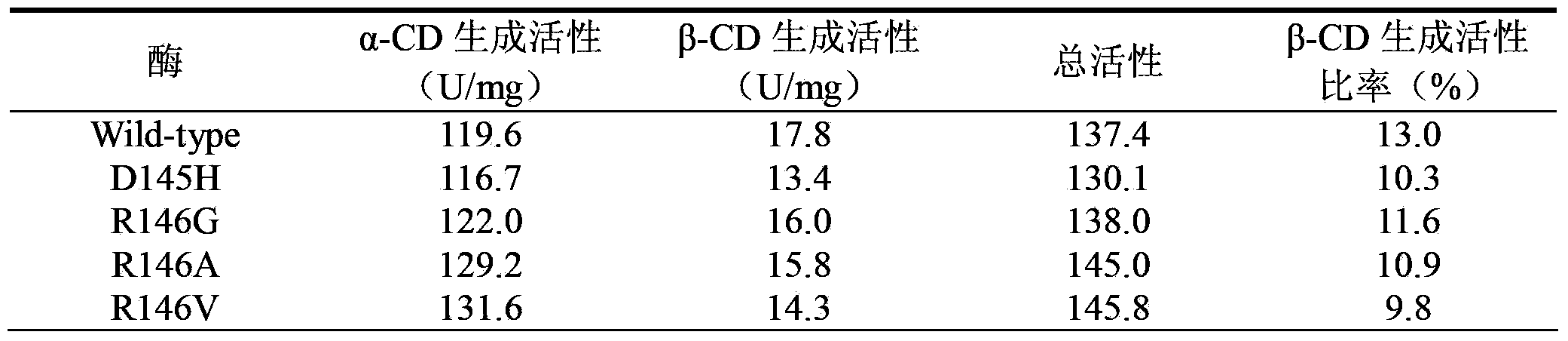
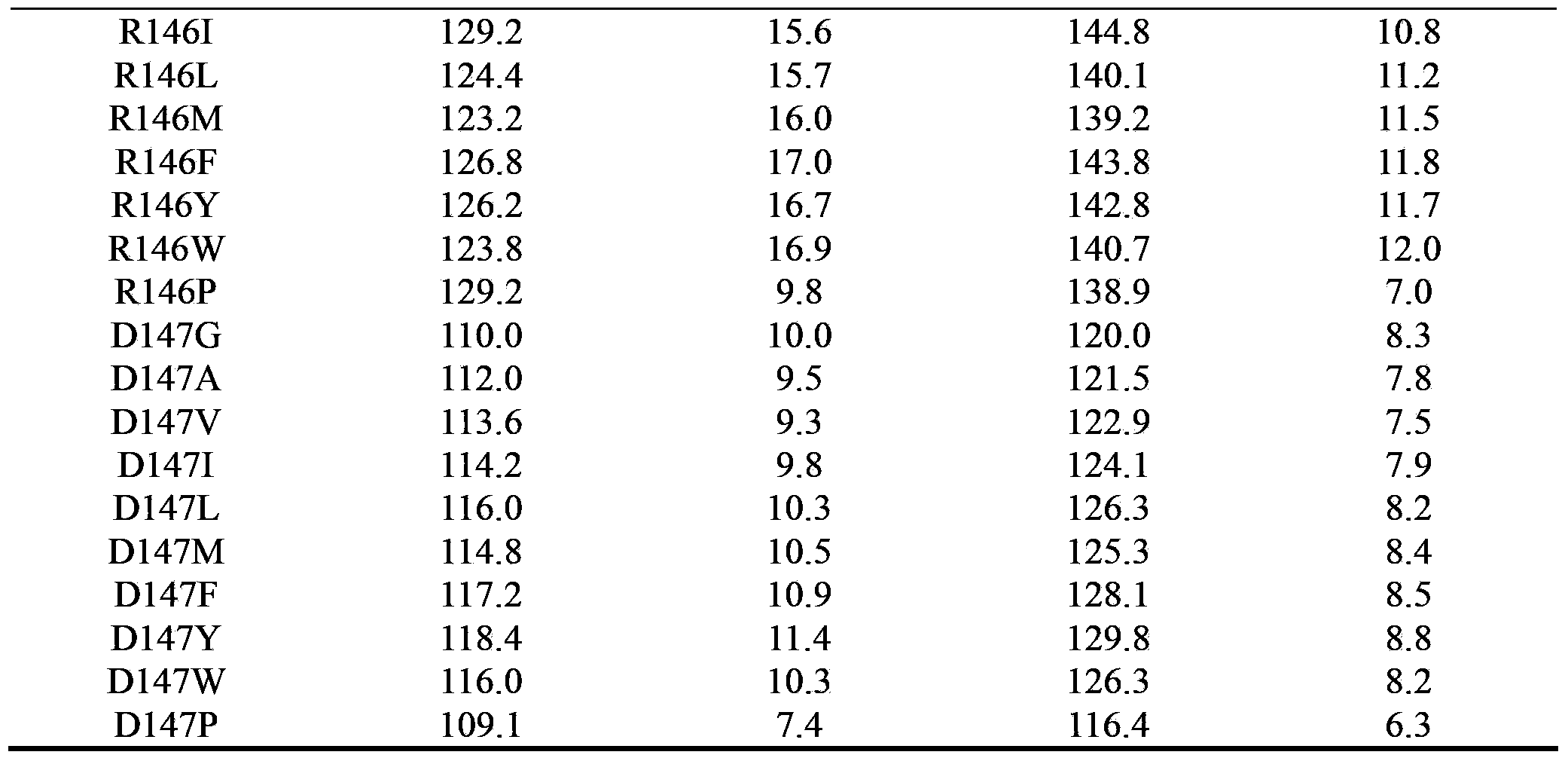
Cyclodextrin glucosyltransferase mutant for high-specificity production of alpha-cyclodextrin
A glucose-based, mutant technology, applied in the field of enzyme engineering, can solve the problems of reducing the yield of CGTase, increasing the ratio of β-cyclodextrin, increasing the difficulty of separation and purification, etc., achieving a wide range of industrial application prospects, enhancing activity, and reducing production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 This example illustrates the preparation of mutant and wild enzymes.
[0026] 1) Site-directed mutation
[0027] Using the plasmid ompA-PET20b(+) / cgt (see: Li Bin, Wu Jing, Chen Jian. Effect of signal peptide on extracellular expression of Paenibacillus macerans α-cyclodextrin glucosyltransferase in Escherichia coli[J ].Industrial Microbiology, 2011,41(3):54-59; Paenibacillus macerans JFB 05-01CCTCC NO:M 208063) as a template, using synthetic mutant primers, for overlapping PCR.
[0028]The PCR reaction system is: 5×PS buffer 10μL, dNTPs Mix (2.5mM) 4μL, forward primer (10μM) 1μL, reverse primer (10μM) 1μL, template DNA 1μL, Prime STAR HS DNA polymerase (5U / μL) 0.5 μL, add double distilled water to 50 μL. PCR amplification conditions were: pre-denaturation at 94°C for 4 min; followed by 30 cycles (98°C for 10 s, 58°C for 5 s, 72°C for 6 min); 72°C for 10 min. The PCR product was digested with Dpn I (Fermentas company), and transformed into Escherichia coli ...
Embodiment 2
[0033] Example 2 This example illustrates an enzyme activity assay.
[0034] Enzyme activity assay method:
[0035] Method for measuring α-cyclization activity by methyl orange method: Take 0.1 mL of appropriately diluted enzyme solution and add 2.0 mL of 1% (w / v) soluble starch solution prepared in advance with 50 mM phosphate buffer (pH 5.5) Medium (substrate preheated at 45°C for 10min), after reacting at 45°C for 10min, add 0.2mL of 3.0M hydrochloric acid to terminate the reaction, then add 0.2mL of 0.44mM methyl orange, incubate at 16°C for 15min, under 505nm Measure the absorbance. One enzyme activity unit is defined as the amount of enzyme required to generate 1 μmol α-cyclodextrin per minute under the above conditions.
[0036] The method for the determination of β-cyclization activity by phenolphthalein method: take 0.1mL of appropriately diluted enzyme solution and add it to 2.0mL of 1% (w / v) soluble starch solution prepared in advance with 50mM phosphate buffer (p...
Embodiment 3
[0037] Example 3 This example illustrates the HPLC method for analyzing the amount of cyclodextrin produced.
[0038] Prepare 5% (w / v) soluble starch solution (pH5.5) as the substrate. A certain amount of CGTase wild enzyme and mutant enzyme were added respectively, the amount of enzyme added was 4U / g starch, and placed at 45°C and 150rpm to react for 10h. Sampling, boiled in boiling water for 10 minutes to inactivate the enzyme, mixed 500 μL with 95% (V / V) ethanol 1:1, left at room temperature for 2 hours to precipitate large molecular weight dextrin or limit dextrin, centrifuged at 12000 rpm for 20 minutes, and took the supernatant for HPLC analysis.
[0039] The chromatographic condition that adopts HPLC to carry out product analysis is: Agilent 1200HPLC chromatograph, Agilent autosampler, chromatographic column Thermo Aps-2 HYPERSIL (4.6mm * 250mm), Agilent 2410 differential detector; Mobile phase (V / V) is 70 % acetonitrile aqueous solution, flow rate 0.8mL min -1 ; Colu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com