Hair-like hydrophilic polymer hybridization magnetic nanoparticle immobilized enzyme and preparation method thereof
A technology of immobilized enzymes and magnetic nanoparticles, applied in the direction of immobilization on/in organic carriers, fermentation, etc., can solve problems such as sample loss, affecting the sensitivity and quantitative accuracy of protein identification, and limiting enzymatic hydrolysis efficiency. To achieve the effect of increasing the solid loading capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
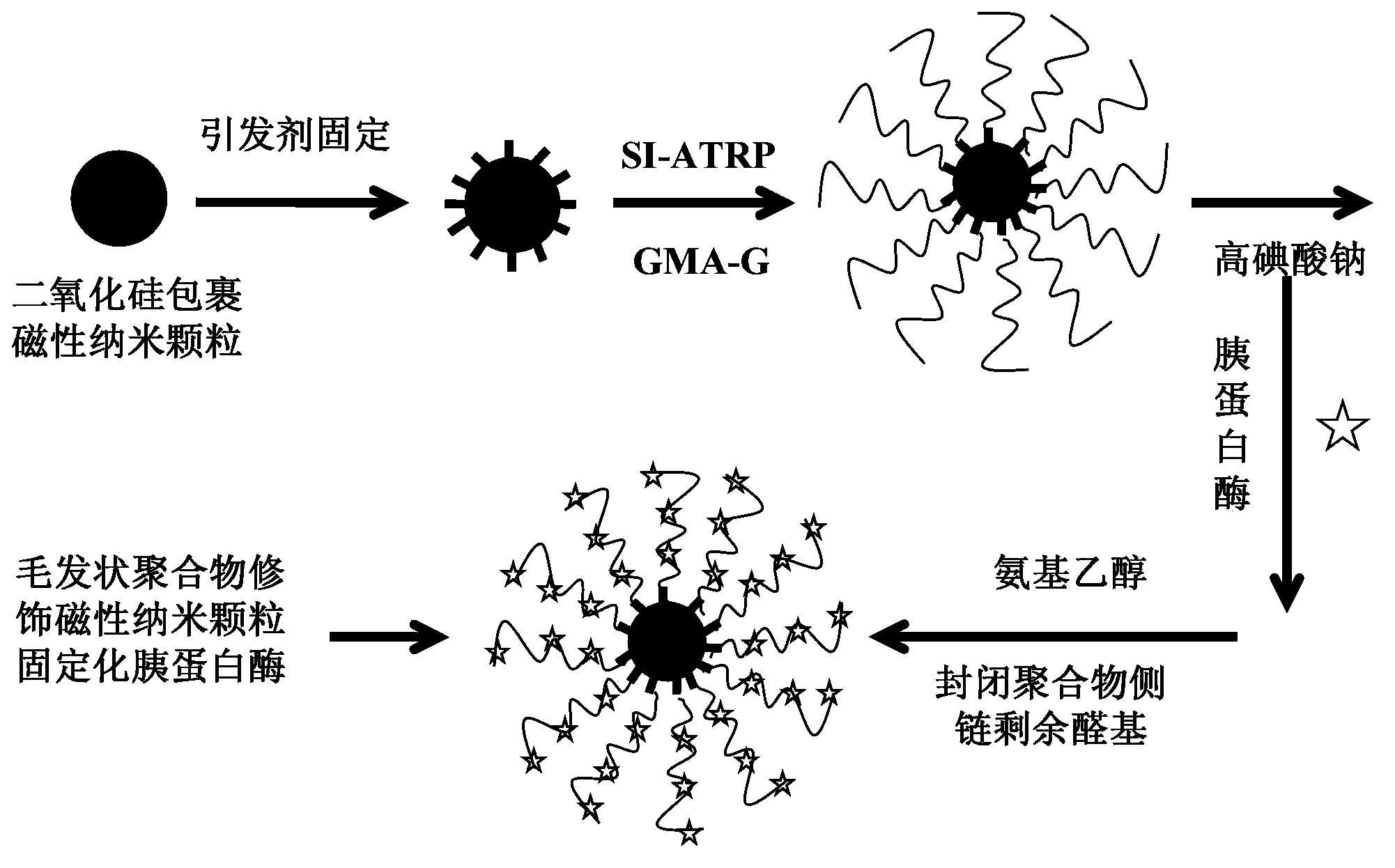
[0045] Example 1. Preparation of Hair-like Hydrophilic Polymer Hybrid Magnetic Nanoparticles Immobilized Protease
[0046] according to figure 1 The process flow shown was carried out.
[0047] 1.1 Synthesis of SI-ATRP initiator. Using 3-aminopropyltriethoxysilane and 2-bromoisobutyryl bromide to synthesize SI-ATRP with one end as a silane coupling agent capable of binding to the surface of silica-coated magnetic nanoparticles and the other end as an ATRP initiator Initiator. The details are as follows: 8mmol 3-aminopropyltriethoxysilane and 10mmol triethylamine were added to 12.5ml tetrahydrofuran, after mixing, nitrogen gas was introduced to remove oxygen while ice bathing was carried out for 30min, and then 10mmol 2-bromoisobutyryl bromide was slowly Add it dropwise into the mixture and stir vigorously for 4 hours (continuous nitrogen flow), and finally filter the solution and vacuum-dry it to 1 / 3 of the original volume to remove tetrahydrofuran and triethylamine, remove...
Embodiment 2
[0057] An appropriate amount of bovine serum albumin (BSA) standard protein (its sequence is shown in sequence 1) was weighed and dissolved in 50 mM ammonium bicarbonate solution (pH=8.0), with a final concentration of 2 μg / μl. Add 10 mM mercaptoethanol (DTT), according to the molar ratio of the final concentration of IAA and DTT is 5:1, add IAA solution, and place in dark place for 1 hour to denature the protein. Mix 50 μl of the denatured protein solution (concentration 2 μg / μl) with 10 mg of trypsin immobilized on hydrophilic polymer hybrid magnetic particles, place it in a water bath at 37°C for 1 minute, and then use a magnet to adsorb the enzyme immobilized on magnetic particles to the tube. wall, and the protein hydrolyzate in the supernatant was removed. Take the same amount of denatured protein solution, add trypsin at a mass ratio of 1:50 (trypsin: protein substrate) according to the conventional enzymatic hydrolysis conditions, place it in a water bath at 37°C for 1...
Embodiment 3
[0059] An appropriate amount of bovine serum albumin (BSA) standard protein was weighed and dissolved in 50 mM phosphate buffer (pH=7.8), with a final concentration of 2 μg / μl. Add 10 mM mercaptoethanol (DTT), according to the molar ratio of the final concentration of IAA and DTT is 5:1, add IAA solution, and place in dark place for 1 hour to denature the protein. Take 50 μl of the denatured protein solution (concentration 2 μg / μl) and mix it with 10 mg of hydrophilic polymer hybrid magnetic particle-immobilized endoproteinase Glu-C, place it in a water bath at 37°C for 1 minute and fix the magnetic particle with a magnet The enzyme is adsorbed on the tube wall, and the protein hydrolyzate in the supernatant is taken out. Take the immobilized enzyme hydrolyzate and spot it on the target of the matrix-assisted laser desorption ionization time-of-flight mass spectrometer and let it air-dry naturally, then spot the CHCA matrix (5 mg / ml, dissolved in 50% acetonitrile, 0.1% TFA), a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com