Synthetic method of glycopeptide
A synthesis method and glycopeptide technology, which is applied in the field of glycopeptide synthesis, can solve the problems of small loading capacity of solid phase carriers, limit the large-scale synthesis of glycopeptides, and low activity, and achieve large-scale synthesis, heterogeneous separation, and high reaction efficiency. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
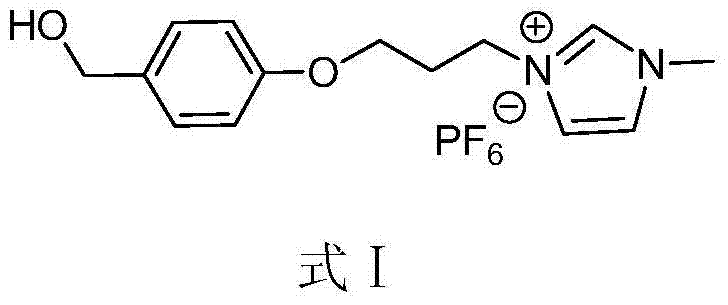
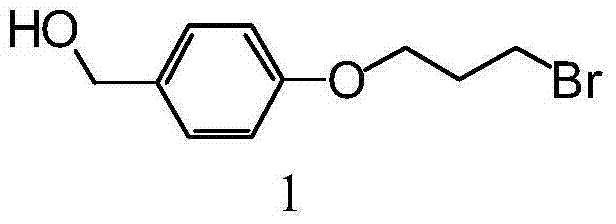
[0040] Embodiment 1, the synthesis of ionic liquid
[0041] The reaction equation is as follows:
[0042]
[0043] Synthesis of Compound 1: Under nitrogen protection, 4-hydroxymethylphenol (4.00 g, 32.22 mmol), potassium carbonate (4.67 g, 33.82 mmol) and 1,3-dibromopropane (9.85 ml, 96.44 mmol ) in dry acetone (30 mL) was stirred at reflux for 5 hours. After thin-layer chromatography showed that 4-hydroxymethylphenol was consumed, the reaction solution was filtered and the solvent was evaporated under reduced pressure, and the resulting mixture was separated and purified by column chromatography (ethyl acetate:petroleum ether=1:3→1:2) , to obtain compound 1 (3.28 g, 39.6%) as a white solid.
[0044] The characterization results of compound 1 are as follows: 1 H NMR (500MHz, CDCl 3 )δ7.29(d,J=8.5Hz,2H),6.90(d,J=8.5Hz,2H),4.62(s,2H),4.11(t,2H),3.61(t,2H),2.32( m,2H); 13 C NMR (150MHz, CDCl 3 )δ158.4, 133.5, 128.7, 114.7, 65.5, 65.0, 32.4, 30.1.
[0045] Synthesis of ...
Embodiment 2
[0047] Embodiment 2, the synthesis of glycosylated amino acid building block formula III
[0048] The reaction equation is as follows:
[0049]
[0050] In the above reaction equation, the meanings of the abbreviations used are as follows: Troc: 2,2,2-trichloroethoxycarbonyl; TBSCl: tert-butyldimethylsilyl chloride; TBS: tert-butyldimethylsilyl; Ac : acetyl; Fmoc: 9-fluorenylmethoxycarbonyl; Ser: serine; Allyl: allyl; DMF: N,N-dimethylformamide; DCM: dichloromethane; DBU: 1,8-diazepine Bicycloundec-7-ene.
[0051] "One-pot" synthesis of glycosyl donor 3: compound 2 (12.0 g, 33.84 mmol) was dissolved in pyridine (150 ml) solvent, stirred and cooled to 0 °C, tert-butyldimethylsilyl chloride (6.63 g, 44.00 mmol) was added to the reaction system, and after 30 minutes, it was stirred overnight at room temperature. After thin-layer chromatography showed that compound 5 was consumed, acetic anhydride (35 ml) was added to the reaction system, stirred at room temperature for 6 hour...
Embodiment 3
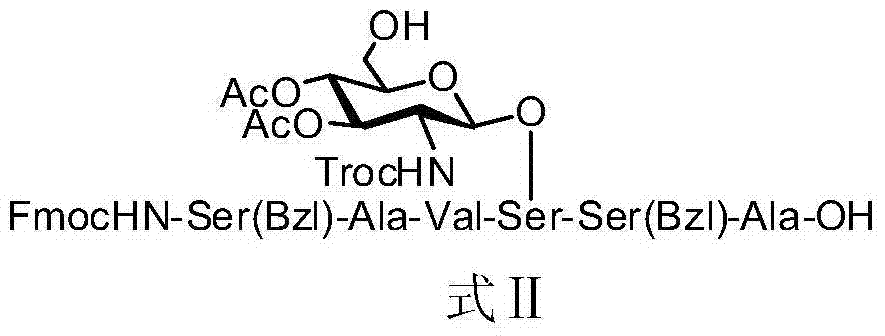
[0057] Embodiment 3, the glycopeptide shown in synthetic formula II
[0058]
[0059] In the above process, the reaction conditions of each step are as follows: (a) 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDCI), 4-dimethylaminopyridine (DMAP), Acetonitrile, nitrogen, room temperature, 7 hours; (b) piperidine, dichloromethane, room temperature, 0.5 hours; (c) benzotriazole-N,N,N',N'-tetramethyluronium hexafluorophosphate salt (HBTU), 1-hydroxybenzotriazole (HOBt), N,N-diisopropylethylamine (DIPEA), acetonitrile, nitrogen, room temperature, 8 hours; (d) trifluoroacetic acid: water = 95: 5 (v / v), dichloromethane, 1 hour.
[0060] In the following preparation, the heterogeneous separation method used is as follows:
[0061] For the purification of ionic liquid-supported compounds. The specific operation process is as follows: the reaction mixture is dissolved in dichloromethane (10 ml / g), 4 times the volume of isopropyl ether is added, and then the solven...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com