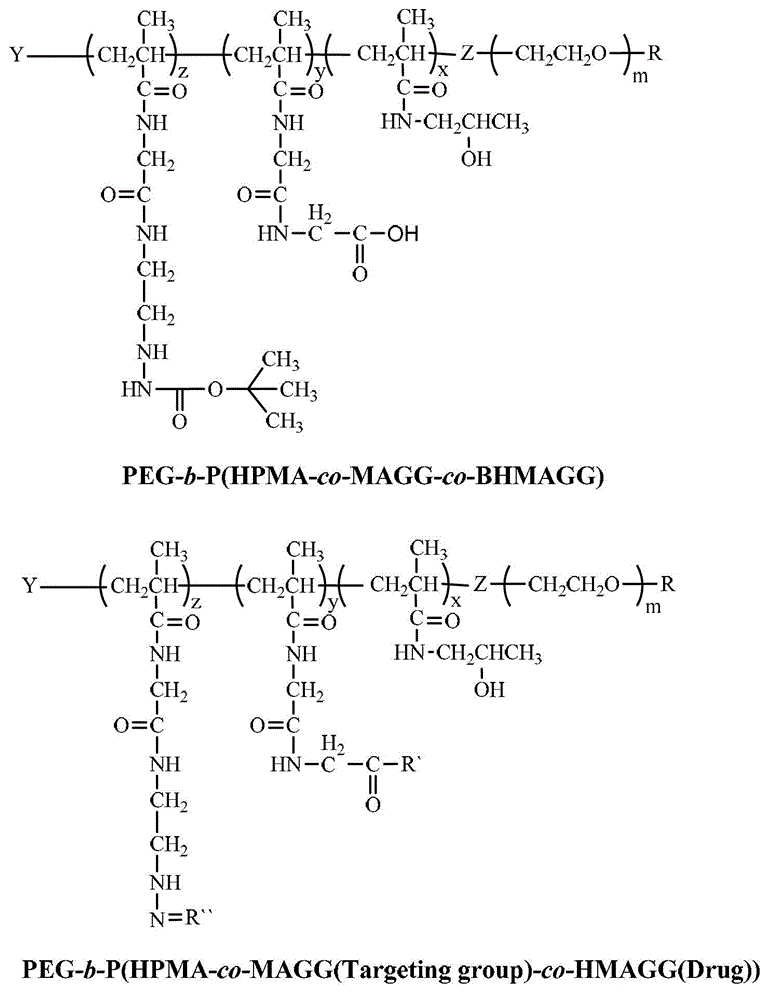
Novel efficient anti-tumor targeting drug carrier and preparation method thereof
A polymer chain and monomer polymerization technology, which is applied in the direction of antineoplastic drugs, drug combinations, and pharmaceutical formulations, can solve problems such as wide molecular weight distribution, single function, and uncontrollable molecular weight, and achieve high targeting, excellent performance, and The effect of good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1, preparation PEG 5k -b-P(HPMA 30k -co-MAGG 5k -co-BHMAGG 8k )
[0050] Dissolve 2.4g of single-terminal hydroxyl or amino PEG with a number average molecular weight of 5000 in 40mL of dry toluene, add 0.68g of CPAD and 0.041g of DMAP, add 0.74g of DCC after complete dissolution, and stir at room temperature for 96 hours. Suction filtration, pour the filtrate into excess diethyl ether, and then suction filtration, the resulting precipitate was dissolved in a small amount of toluene, and then precipitated with diethyl ether, and this was repeated three times. The precipitate was vacuum-dried at 40° C. for 24 hours, and the obtained product was a macromolecular chain transfer agent of PEG.
[0051] Take 52mg of macromolecular chain transfer agent, 312.5mg of HPMA, 52mg of MAGG, 83mg of BHMAGG, 1mg of 4,4'-azobis(4-cyanovaleric acid), and 5mL of DMSO in a Shrek tube, vacuumize under refrigeration, and then recover Return to room temperature, fill with argo...
Embodiment 2
[0053] Embodiment 2, preparation PEG 5k -b-P(HPMA 30k -co-MAGG 5k (Folate)-co-BHMAGG 8k )
[0054] Folate-NH 2 Preparation: 4.41g of folic acid and 10-fold excess of ethylene glycol bis-2-aminoethyl ether were reacted at 35°C for 48 hours in anhydrous DMSO as a solvent under the catalyst of EDC and NHS equivalent to folic acid. After the reaction was completed, the reaction solution was poured into water for precipitation. The filtrate was collected and freeze-dried, and the product was purified by semi-preparative liquid chromatography (Shimadzu LC-6AD, Japan).
[0055] PEG 5k -b-P(HPMA 30k -co-MAGG 5k (Folate)-co-BHMAGG8k ) preparation: the PEG prepared in Example 1 5k -b-P(HPMA 30k -co-MAGG 5k -co-BHMAGG 8k ) and Folate-NH 2 With anhydrous DMSO as solvent, it is prepared under the catalysis of EDC and NHS.
[0056] The specific steps are: take 0.5g PEG 5k -b-P(HPMA 30k -co-MAGG 5k -co-BHMAGG 8k ) was dissolved in 20ml of anhydrous DMSO, 0.3g of EDC and 0.1...
Embodiment 3
[0057] Example 3, PEG 5k -b-P(HPMA 30k -co-MAGG 5k (Folate)-co-HMAGG 8k (Dox)) preparation
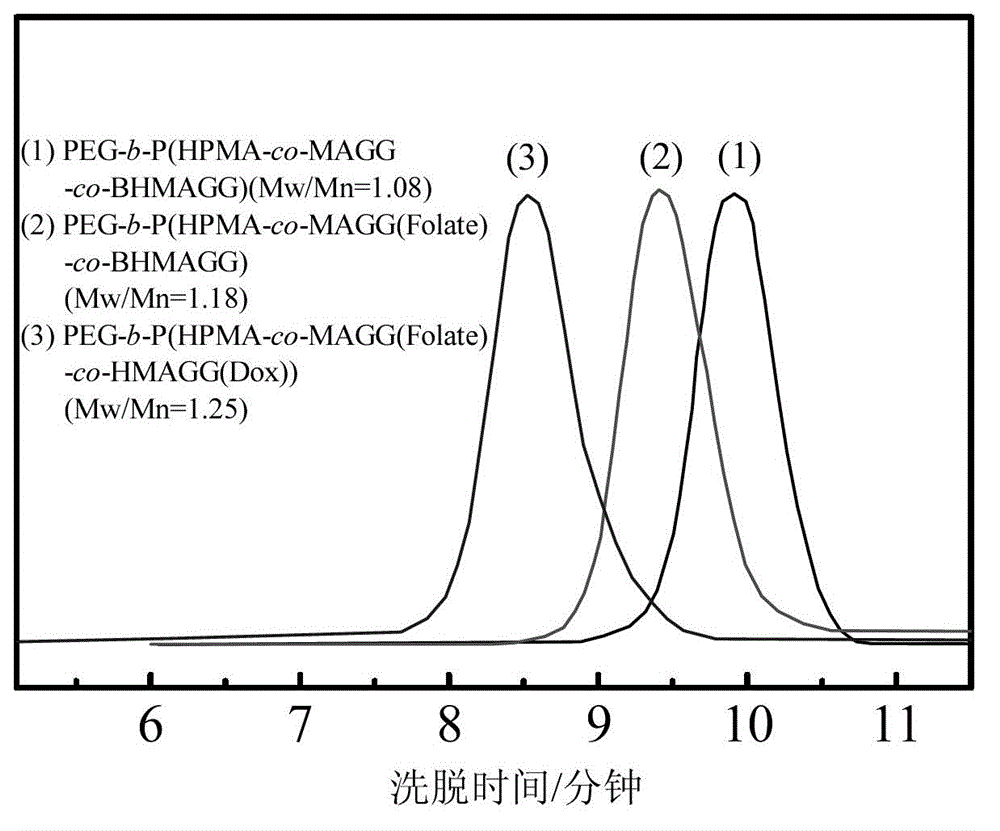
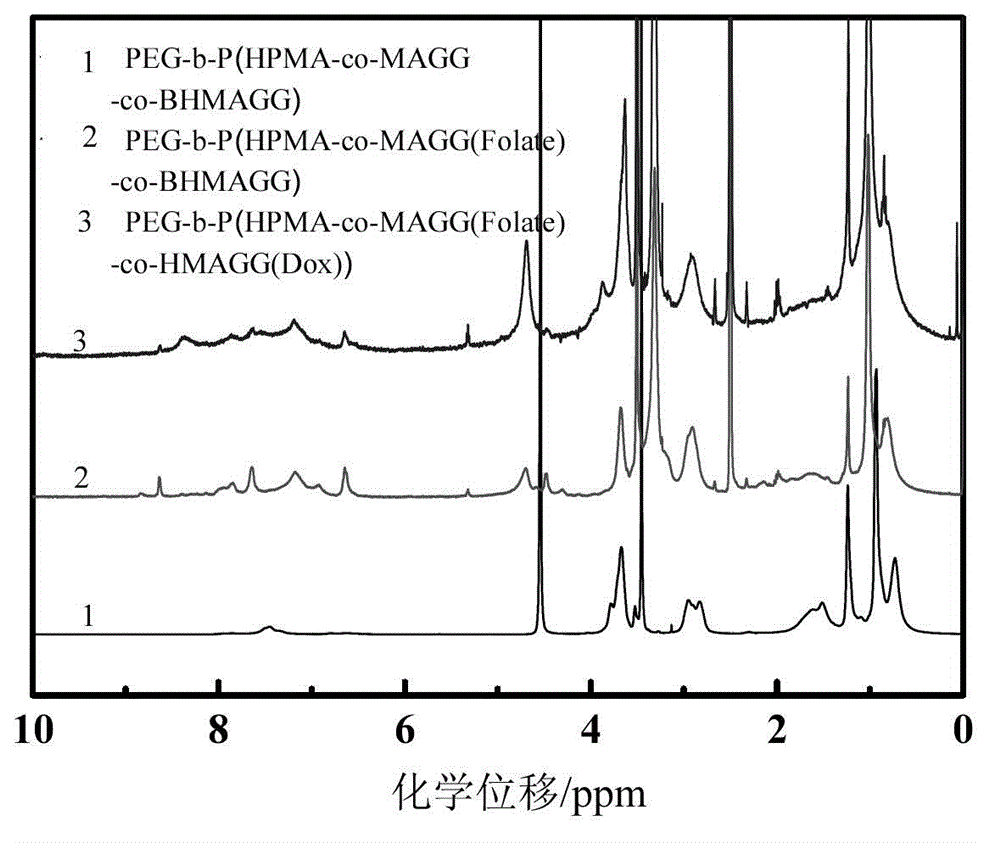
[0058] The PEG prepared in 100mg embodiment 2 5k -b-P(HPMA 30k -co-MAGG 5k (Folate)-co-BHMAGG 8k ) first remove the Boc protection in 15ml of pure trifluoroacetic acid, the reaction time is 30 minutes, after the reaction is completed, the trifluoroacetic acid is removed by rotary evaporation to obtain PEG 5k -b-P(HPMA 30k -co-MAGG 5k (Folate)-co-HMAGG 5.4k ). The product was dissolved in 20ml of anhydrous DMSO, 100mg of Dox and 0.2ml of glacial acetic acid were added, and reacted at 37°C for 48 hours. After the reaction was completed, the resulting reaction solution was purified with a gel chromatographic column, and the characterization results were as follows: figure 2 with image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com