Recombined lipoxygenase and preparation method thereof
An oxygenase and recombinant lipid technology, applied in the field of bioengineering, can solve the problems of many types of enzymes, difficult to control, unstable enzyme activity, etc., and achieve the effects of high enzyme purity, low degree of evolution, and stable enzyme activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: Cloning of PhLOX full-length cDNA sequence
[0019] Extract the genomic DNA of Porphyra thallus with the CTAB method (cetyltrimethylammonium bromide method), and the steps are as follows:
[0020] 1) After preheating 2% CTAB extract in a water bath at 65°C, dispense 15mL of the extract into 50mL centrifuge tubes, add 0.1% mercaptoethanol, mix well and set aside;
[0021] 2) Take 500mg of fronds, quickly grind them into powder with liquid nitrogen in a sterile mortar, and immediately transfer them to CTAB extract;
[0022] 3) After oscillating evenly, heat in a 65°C water bath for 60 minutes, during which time the centrifuge tubes are repeatedly inverted several times every 10 minutes;
[0023] 4) Put it into a centrifuge for centrifugation, the centrifugation conditions are: 4°C, 8000rpm, 10min, after the centrifugation, suck the supernatant;
[0024] 5) Add an equal volume of phenol / chloroform / isoamyl alcohol mixture (ratio: 25 / 24 / 1) to the above supern...
Embodiment 2
[0047] Example 2: Construction of recombinant system for PhLOX prokaryotic expression
[0048] Design a pair of specific primers with NdeI / HindIIII restriction sites at both ends of the ORF (open reading frame) of PhLOX: Forward primer: 5'GGAATTCCATATGATGGGGAATGCG3'
[0049] Reverse primer: 5'CCCAAGCTTCTAGATGTCGATGGACAG3'.
[0050]The PhLOX complete ORF was amplified using the total thallus cDNA as a template. The cloned target fragment was first connected to pMD-18T (Takara, Japan) to form a LOX-18T recombinant cloning vector, and sequenced by transforming E.coli DH5α to check the correctness of the coding of the target fragment. Select pET-28a (Takara, Japan) as the expression vector, and in each 40 μL system, take the LOX-18T and pET-28a empty vectors and use 2U of NdeΙ / HindΙΙΙ (BioLabs, UK) for double enzyme digestion, and incubate at 37°C for 2h above. Take 5 μL of the digested product and perform 1.5% agarose gel electrophoresis for detection. After the plasmid is dig...
Embodiment 3
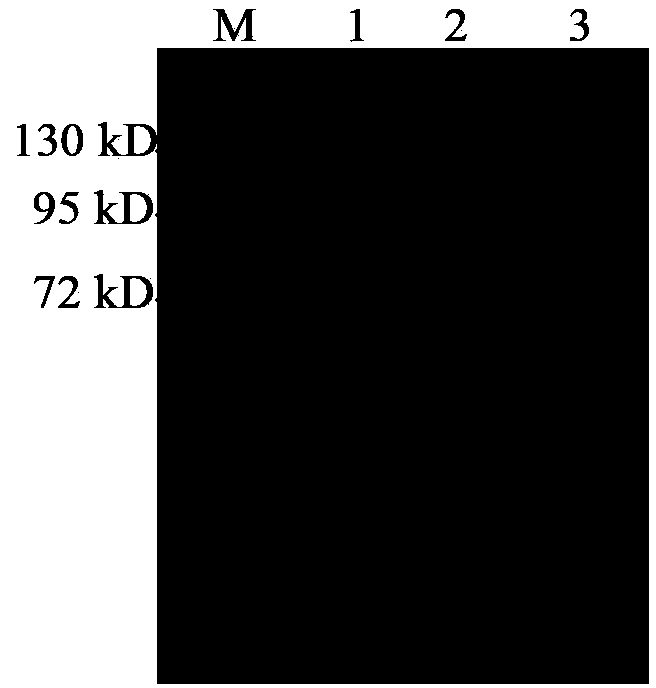
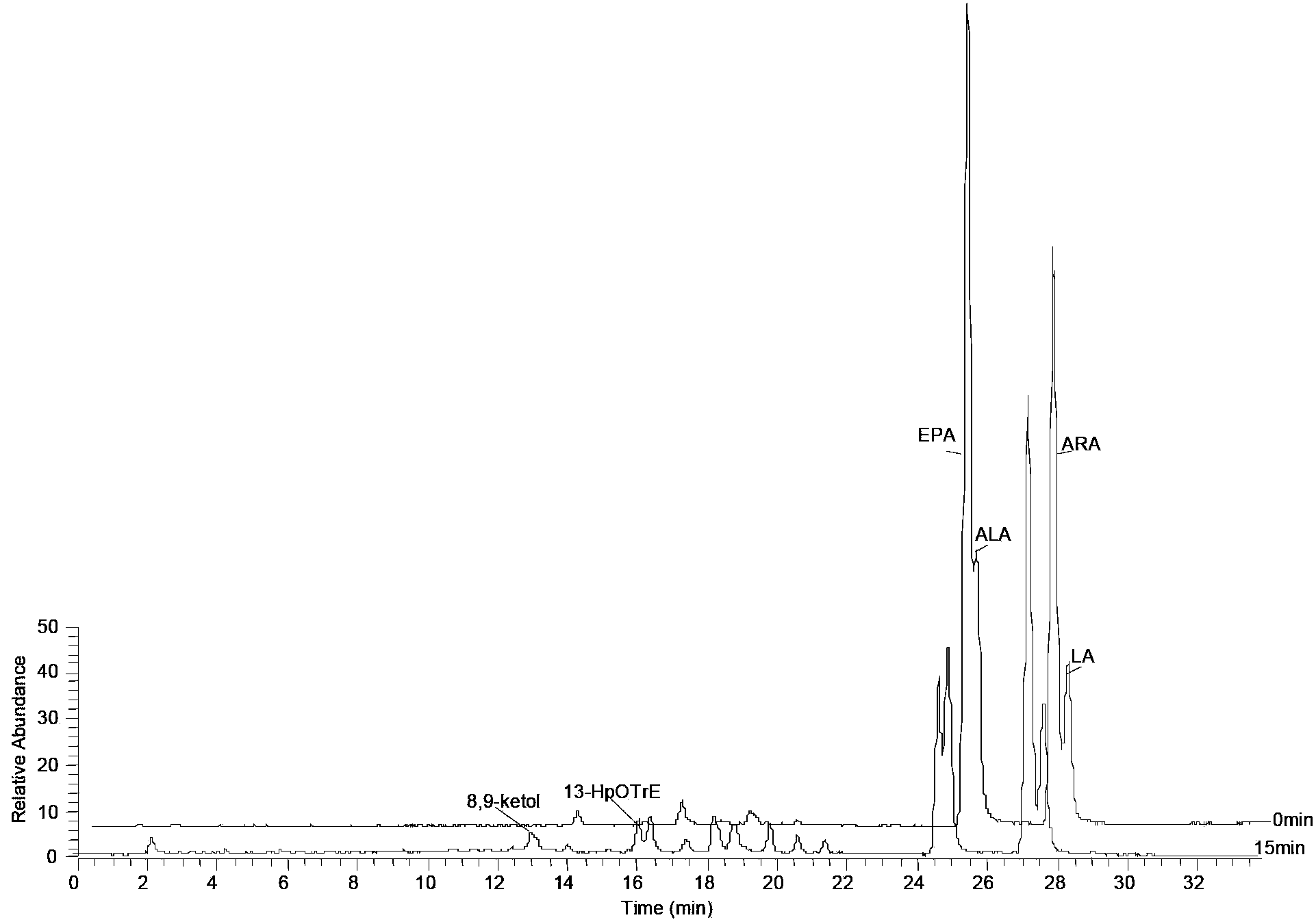
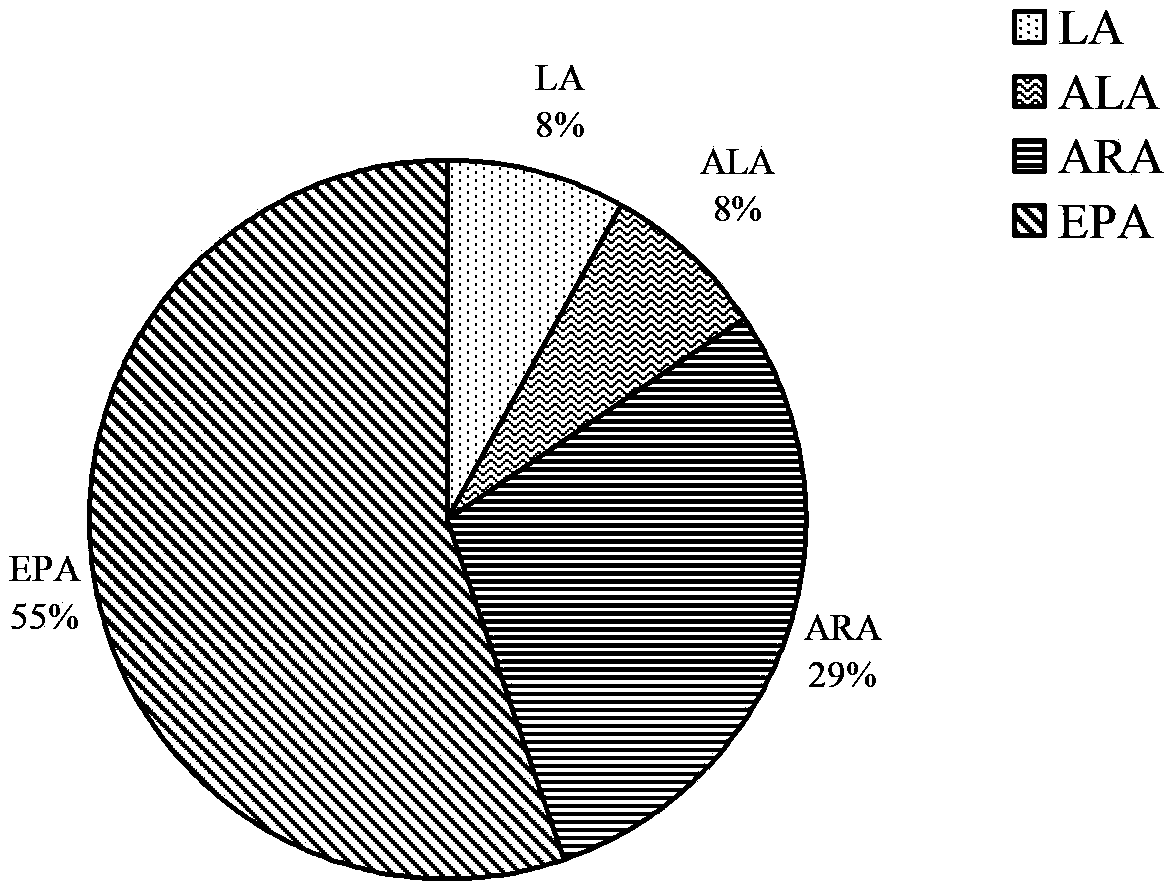
[0051] Example 3: Induction and purification of PhLOX protein
[0052] The cloning strain obtained in Example 2 was activated, expanded, and expressed with 0.1 mM IPTG. The induction conditions are 20°C, 100rpm, 14-20h. After the induction was completed, the bacterial cells were collected by centrifugation at 5000 rpm, 4°C, and 15 min. Remove the supernatant culture solution, and then fully resuspend the cells with 10mL cell lysate buffer A-II (50mM Tris-HCl, pH8.0, 200mM NaCl, 10% (v / v) glycerol, 0.1% tween20). The cells were crushed and homogenized (Bertin technologies Precellys24Dual, France) at 6500rpm, 20s-breaking / 2min-ice-bath program, with 4 cycles. The homogenate was centrifuged at 12000 rpm at 4°C for 10 min, and the supernatant was collected.
[0053] The target protein was purified from the supernatant using 6×His-Tagged Protein Purification Kit (CWBIO, China), and the target protein was collected by gradient elution with eluents containing 10mM, 50mM, 150mM, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com