Method for coproducing cyclohexanol and alkanol
A cyclohexane and cyclohexanol technology, applied in the field of co-production of cyclohexanol and alkanol, can solve the problems of increased energy consumption, slow rate, low reaction efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
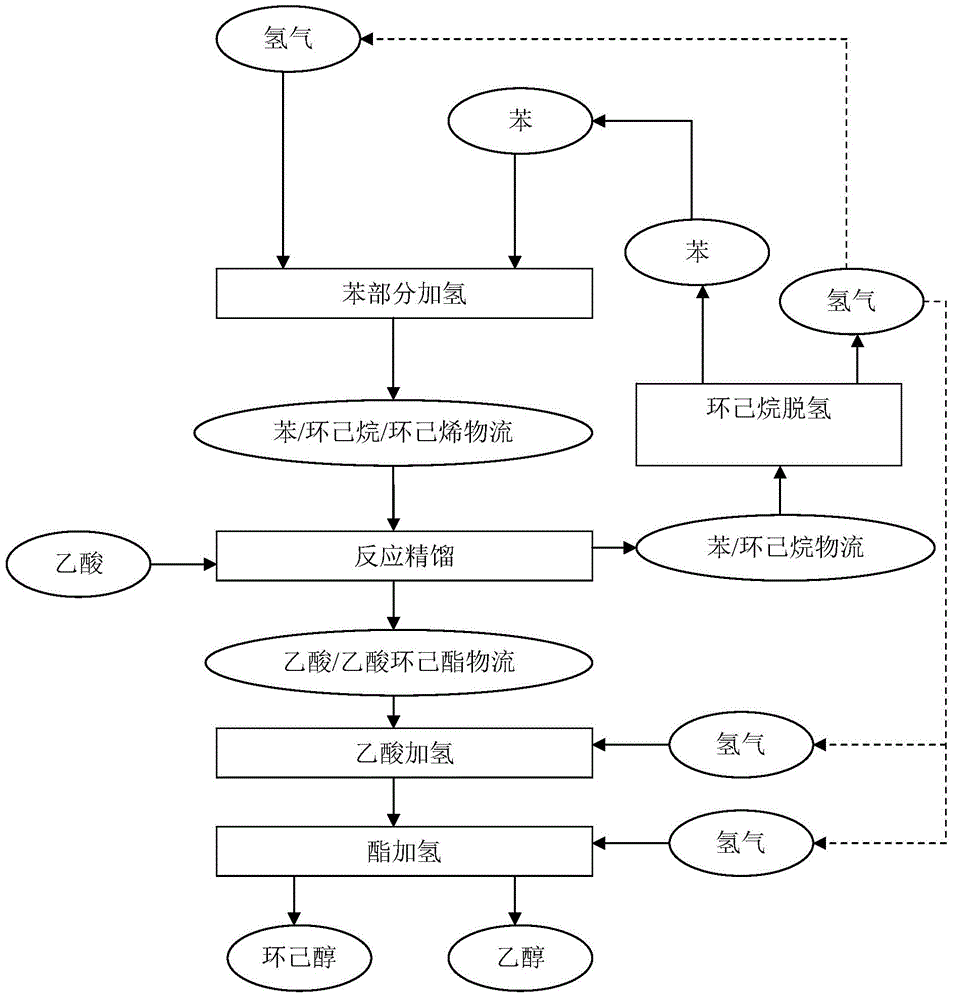
[0131] This example is used to illustrate the method for producing cyclohexene source through partial hydrogenation of benzene.
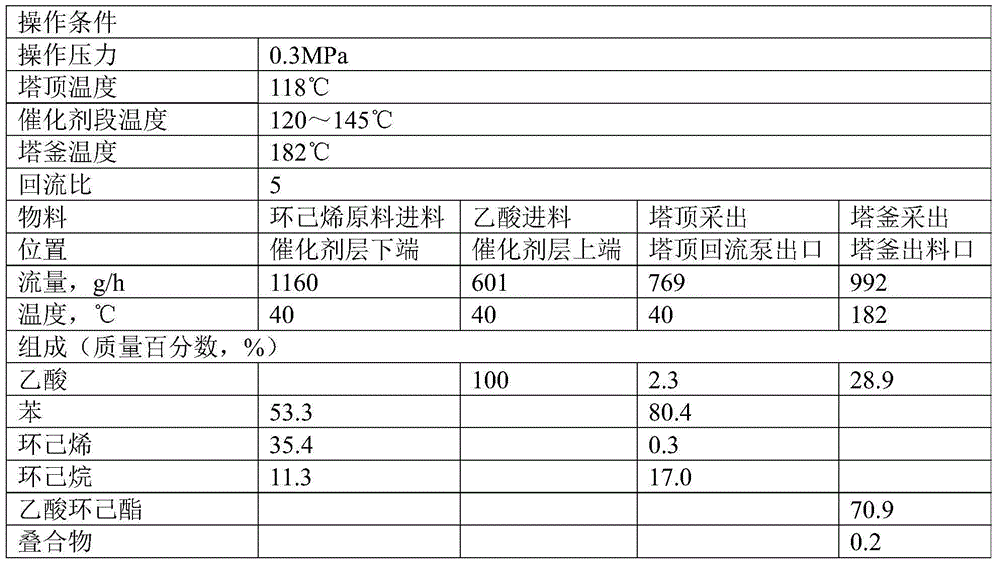
[0132] Benzene and hydrogen are injected into the hydrogenation reactor filled with ruthenium particle catalyst at a molar ratio of 1:3, and the benzene hydrogenation reaction is carried out under the conditions of a reaction temperature of 135°C, a pressure of 4.5MPa, and a residence time of 15 minutes, and the reaction product is separated After hydrogen evolution, the liquid product was collected. Continuous operation 1000h. After the test, gas chromatographic analysis was performed on the collected liquid products, and the composition was 53.3% of benzene, 35.4% of cyclohexene, and 11.3% of cyclohexane in terms of mass percentage.
Embodiment 2
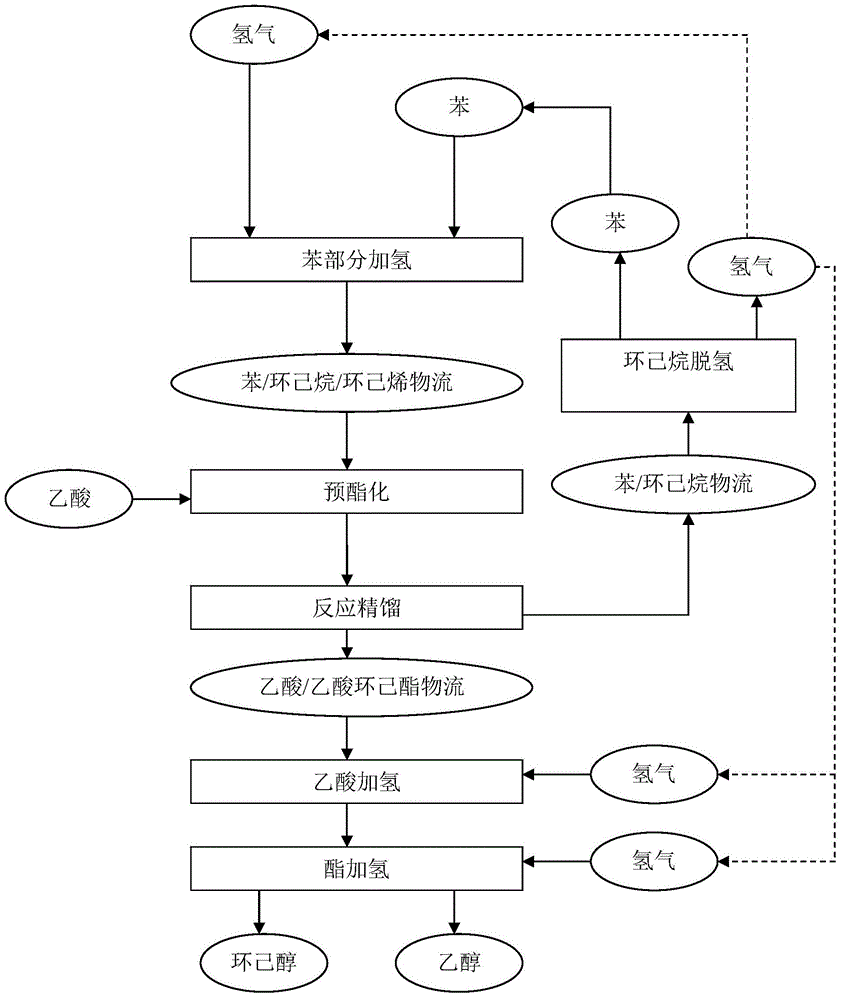
[0136] This example is used to illustrate the method of esterifying acetic acid with cyclohexene / cyclohexane / benzene.
[0137] The high-temperature-resistant sulfonic acid ion exchange resin (the brand name is Amberlyst 45, produced by Rhom&Hass Company) is crushed into a powder with a particle size of less than 200 mesh (0.074mm) with a multi-stage high-speed pulverizer, and pore-forming agents, lubricants, and antioxidants are added. The agent and adhesive are mixed evenly on a high-speed mixer, and then internally kneaded on an internal mixer at 180°C for 10 minutes to make the material completely plasticized, and then injected into a mold to make a Raschig ring with a diameter of 5mm, a height of 5mm, and a wall thickness of 1mm. type resin catalyst filler. Put 1950mL of this catalyst packing into the middle of the model reaction tower (filling height is 1m, equivalent to 8 theoretical trays), and put 1950mL of glass spring packing with a diameter of 3mm and a length of 6m...
Embodiment 3
[0147] This example is used to illustrate the method of esterifying acetic acid with cyclohexene / cyclohexane / benzene.
[0148] The ball type H of φ3~4 0.5 Cs 2.5 PW 12 o 40 / SiO 2 Catalyst (by H 0.5 Cs 2.5 PW 12 o 40 Powder and coarse-porous silica gel powder with a particle size of less than 200 meshes are fully mixed in a mixer, rolled into balls in a sugar coating machine with silica sol as a binder, and then dried and roasted) sandwiched into titanium wire mesh waves In the plate, a cylindrical structured packing with a diameter of 50 mm and a height of 50 mm was made. Put 1L of this packed catalyst into the middle of the model reaction tower (the packing height is 1m, equivalent to 12 theoretical trays), and put 1950mL glass spring packing with a diameter of 4mm and a height of 4mm at the top and bottom (the packing height is 1m, equivalent to at 15 theoretical plates).
[0149] The cyclohexene raw material and acetic acid were pumped into the preheater by the m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| flash point | aaaaa | aaaaa |
| thermal resistance | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com