Method for producing high carbon aldehyde through using olefin hydroformylation reaction
A technology for olefin hydroformylation reaction, which is applied to the preparation of carbon monoxide reaction, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of loss of homogeneous catalyst, decrease in activity, etc. Simple preparation process and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
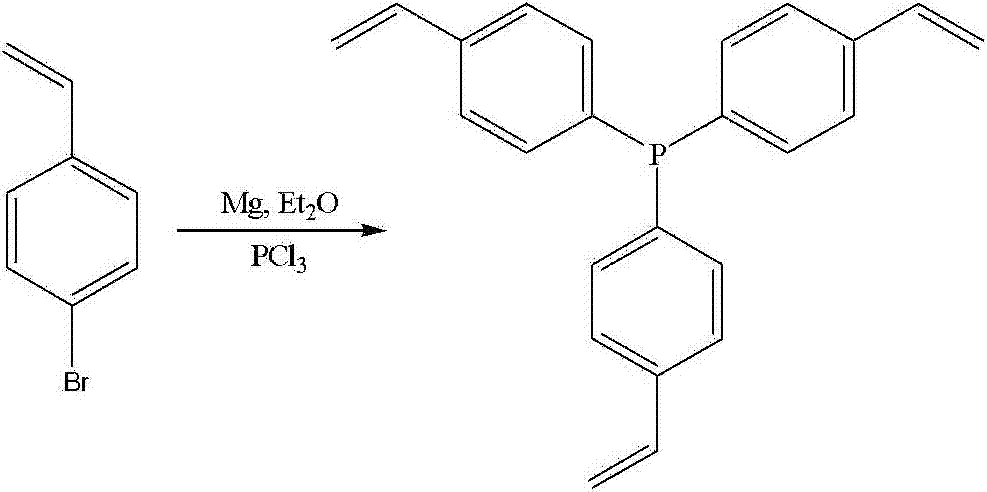
[0055] In an ice-water bath and a nitrogen atmosphere, in a 100mL three-necked round-bottomed flask with a magnetic stirrer, add 0.5g of magnesium powder and 10mL of anhydrous ether in turn, and stir the resulting reaction mixture for 2 hours under these conditions, and then add 4g of A mixed solution of p-bromostyrene and 10mL of anhydrous ether, the resulting reaction mixture was stirred under these conditions for 2 hours, then a mixed solution of 6g of phosphorus trichloride and 10mL of anhydrous ether was added dropwise, and the resulting reaction mixture was stirred under these conditions After 2 hours, add 10 mL of water and stir for 1 hour, then extract the reaction mixture with 90 mL of diethyl ether solution for 3 times, combine the diethyl ether extracts, filter and remove the solvent by rotary evaporation, and the obtained primary product is purified by silica gel column chromatography, which is ready to use Silica gel was used as the stationary phase, and a mixed so...
Embodiment 2
[0057] Under 298K and a nitrogen gas protection atmosphere, 10.0 grams of tris (4-vinylphenyl) phosphine was dissolved in 100.0 ml of tetrahydrofuran solvent, and 1.0 grams of free radical initiator azobisisobutyronitrile was added to the above solution, stirred for 2 Hour. The stirred solution was moved to an autoclave, and polymerized by solvothermal polymerization at 373K under a nitrogen atmosphere for 24 hours. After the above-mentioned polymerized solution is cooled to room temperature, the solvent is vacuumed away at room temperature to obtain a large-surface-area hierarchical porous structure organic ligand polymer carrier formed by tris(4-vinylphenyl)phosphine polymerization. Figure 4 It is a schematic diagram of the technical route of tris(4-vinylphenyl)phosphine ligand polymer carrier polymerization. It is determined by analysis that the degree of polymerization n of the ligand polymer is 450-550, and it has macropores, mesopores and micropores. Hierarchical pore ...
Embodiment 3
[0059] Under 298K and a nitrogen gas protection atmosphere, weigh 3.14 mg of rhodium acetylacetonate dicarbonyl (I) and dissolve it in a three-necked flask of 100.0 ml of tetrahydrofuran solvent, stir and dissolve, add 1.0 g of tris(4-ethylene) prepared in Example 2 The organic ligand polymer formed by the polymerization of phenyl)-based phosphine, the mixture was stirred at 298K and nitrogen gas protection atmosphere for 24 hours, and then the solvent was vacuumed off at room temperature, and the organic ligand polymer self-loaded metal was obtained. Components of solid heterogeneous catalysts. The heterogeneous catalyst prepared above was applied to the hydroformylation reaction of 1-dodecene in a trickle bed. The trickle bed is a stainless steel tube reactor with an inner diameter of 9mm, the catalyst loading is 1.0ml, the synthesis gas pressure is 1.0MPa, and the volume space velocity is 5000h -1 . 1-Dodecene is injected into the reactor with a double plunger micropump, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com