Method for preparing roxithromycin dispersible tablet
A technology of roxithromycin and dispersible tablets, which is applied in the field of roxithromycin tablets and its preparation, can solve the problems of prolonging the wet and hot time of materials, flying dust, and low drying efficiency, so as to improve the bioavailability of drugs and ensure The uniformity of particle quality and the effect of improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
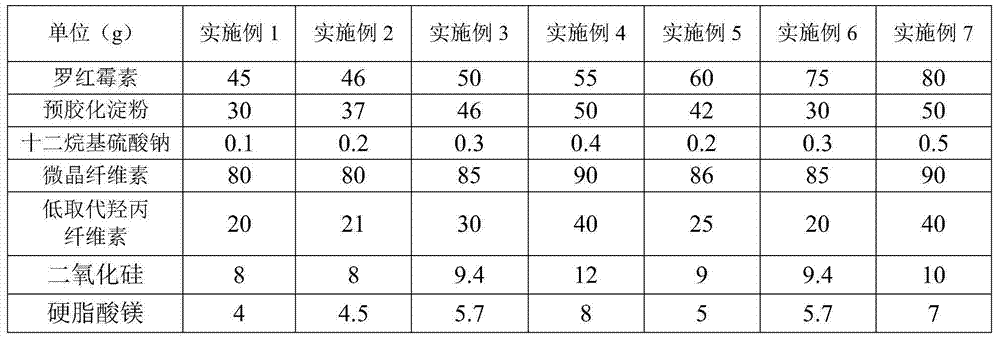
[0044] The preparation raw material ratio of above-mentioned Roxithromycin dispersible tablet, as the preferred implementation situation of Roxithromycin dispersible tablet of the present invention, all have good effect for the storage, transshipment, use etc. of Roxithromycin dispersible tablet Effect improvement should be specially protected.
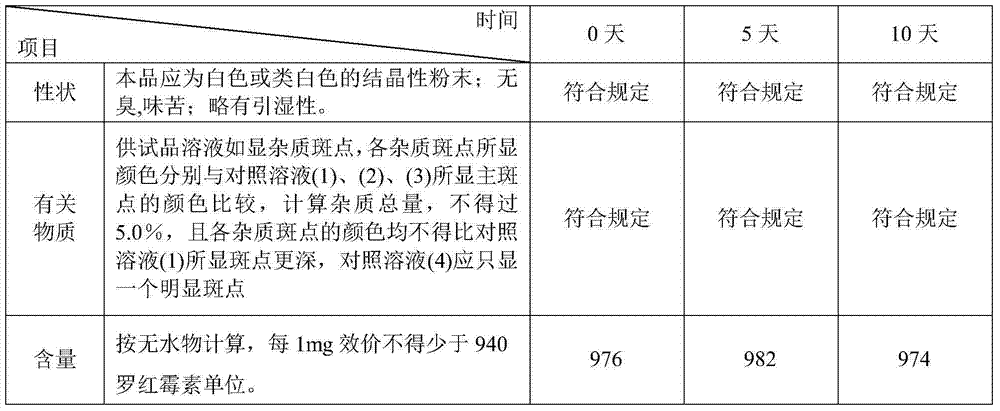
[0045] This product is 9-[O-[(2-methoxyethoxy)-methyl]oxime]erythromycin. Calculated on the basis of anhydrous substance, the potency of roxithromycin per 1mg shall not be less than 940.
[0046] (1) [Properties] This product is white or off-white crystalline powder; odorless, bitter; slightly hygroscopic.
[0047] (2) [Related substances] Take this product, add methanol to make a solution containing 20mg per 1mL, as the test solution; take another standard product of roxithromycin, add methanol to make a solution containing 0.4mg, 0.2 mg, 0.1 mg and 0.04 mg solutions were used as control solutions (1), (2), (3) and (4) respectively...
Embodiment 1
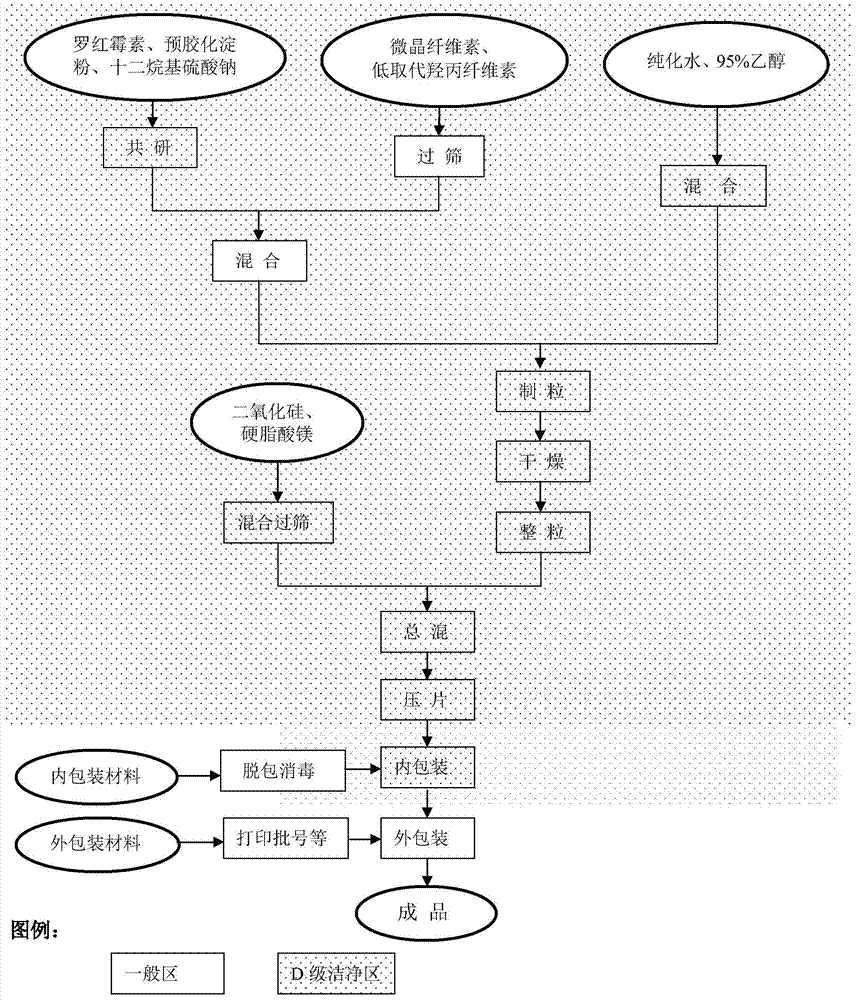
[0051] like figure 1 The preparation process of roxithromycin dispersible tablets shown in Table 1 is to get roxithromycin, pregelatinized starch, sodium lauryl sulfate pre-grinding, cross 100 mesh sieves, get roxithromycin pre-grinding material. Take microcrystalline cellulose and low-substituted hydroxypropyl cellulose and pass through a 80-mesh sieve first, then add roxithromycin pre-ground material and mix evenly, add an appropriate amount of 60% ethanol for wet granulation, dry, and pass through a 30-mesh sieve to obtain a premix pellets. Add silicon dioxide and magnesium stearate as fillers, mix, and compress into tablets to obtain roxithromycin tablets. Carry out internal packaging and external packaging to the obtained tablet sequentially according to the existing conventional process to obtain a finished dispersible tablet.
[0052] Table 1 Roxithromycin Dispersible Tablets Raw Material Consumption Ratio (per 1000 tablets)
[0053]
Embodiment 2-7
[0055] Take raw materials according to the ratio of raw materials in Table 1, pre-grind roxithromycin, pregelatinized starch, sodium lauryl sulfate, press figure 1 The preparation process of the roxithromycin dispersible tablet is shown, and the roxithromycin pre-research material is obtained. Microcrystalline cellulose and low-substituted hydroxypropyl cellulose are then mixed with the above-mentioned roxithromycin pre-ground material, and an appropriate amount of 55vol% ethanol is added, wet granulated, boiled and dried, and passed through a 30-mesh sieve to obtain pre-mixed granules. Add filler silicon dioxide and magnesium stearate to the premixed granules and mix, Ф8.5㎜ shallow concave compression tablet, and make roxithromycin tablets (1000 tablets). Carry out internal packaging and external packaging to the obtained tablet sequentially according to the existing process to obtain a finished dispersible tablet.
[0056] Below according to Chinese Pharmacopoeia second tab...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com