A kind of injectable double cross-linked hyaluronic acid hydrogel and preparation method thereof
A hyaluronic acid and double cross-linking technology, which is applied in the direction of pharmaceutical formulations, medical preparations of non-active ingredients, animal cells, etc., can solve the problems of limiting the practical application of hyaluronic acid, poor controllability of degradation rate, and difficulty in stability , to achieve good microstructure uniformity, excellent biocompatibility, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation of amino / methacryloyl bifunctional hyaluronic acid:
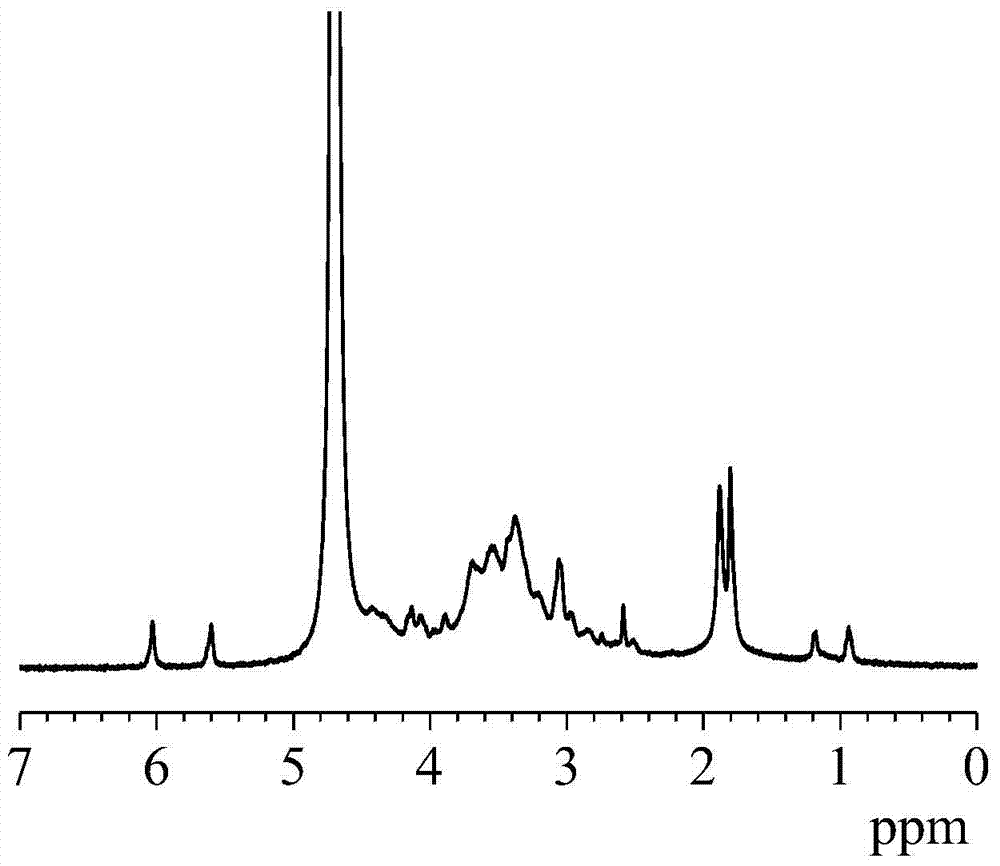
[0034] 1) Prepare an aqueous solution of hyaluronic acid (molecular weight: 600,000) with a concentration of 1% by mass, and then add N-hydroxysuccinimide, 3,3'-dithiodipropionyl hydrazide and 1-(3 -Dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, the amount of which is respectively 0.7, 4 and 0.6 times the number of moles of hyaluronic acid repeating units; then, using a NaOH solution with a concentration of 1mol / L and hydrochloric acid to adjust the pH to 5.2, and stirred and reacted at room temperature for 36 hours; ℃ / 4 hours freeze-drying to obtain amino-functionalized hyaluronic acid;
[0035] 2) Prepare an amino-functionalized hyaluronic acid aqueous solution with a concentration of 1% by mass, then add glycidyl methacrylate with 0.2 times the number of repeating units of hyaluronic acid, and adjust the pH to 8.0, react with magnetic stirring at 70°C for 6 hours, and then dialyze with deion...
Embodiment 2
[0046] Preparation of amino / methacryloyl bifunctional hyaluronic acid:
[0047] 1) Prepare an aqueous solution of hyaluronic acid (molecular weight: 1 million) with a mass percentage concentration of 0.2%, and then add N-hydroxysuccinimide, cystamine and 1-(3-dimethylaminopropyl)-3- Ethylcarbodiimide hydrochloride, its dosage is respectively 0.24, 10 and 0.2 times of the number of moles of hyaluronic acid repeating units; then, the pH is adjusted to 5.0 by using NaOH solution and hydrochloric acid with a concentration of 1mol / L, and at room temperature The reaction was stirred for 24 hours; after the reaction, the reaction system was dialyzed with deionized water for 3 days (the molecular weight cut-off of the dialysis bag was 3500Da, the same below), and the amino functionalized hyaluronic acid;
[0048] 2) Prepare an amino-functionalized hyaluronic acid aqueous solution with a concentration of 0.5% by mass, then add glycidyl methacrylate with 0.15 times the number of repeat...
Embodiment 3
[0054] Preparation of amino / methacryloyl bifunctional hyaluronic acid:
[0055] 1) Prepare an aqueous solution of hyaluronic acid (molecular weight: 800,000) with a mass percentage concentration of 2%, and then add N-hydroxysuccinimide, cystine dimethyl ester and 1-(3-dimethylaminopropyl )-3-ethylcarbodiimide hydrochloride, its dosage is respectively 0.5, 6 and 0.4 times of the number of moles of hyaluronic acid repeating units; then, the pH is adjusted to 5.5, Stir the reaction at room temperature for 60 hours; after the reaction, the reaction system is dialyzed with deionized water for 3 days (the molecular weight cut-off of the dialysis bag is 3500Da, the same below), and freeze-dried at minus 20°C / 72 hours and 20°C / 4 hours Obtain amino functionalized hyaluronic acid;
[0056] 2) Prepare an amino-functionalized hyaluronic acid aqueous solution with a concentration of 1.5% by mass, then add glycidyl methacrylate with 0.4 times the number of repeating units of hyaluronic aci...
PUM
| Property | Measurement | Unit |
|---|---|---|
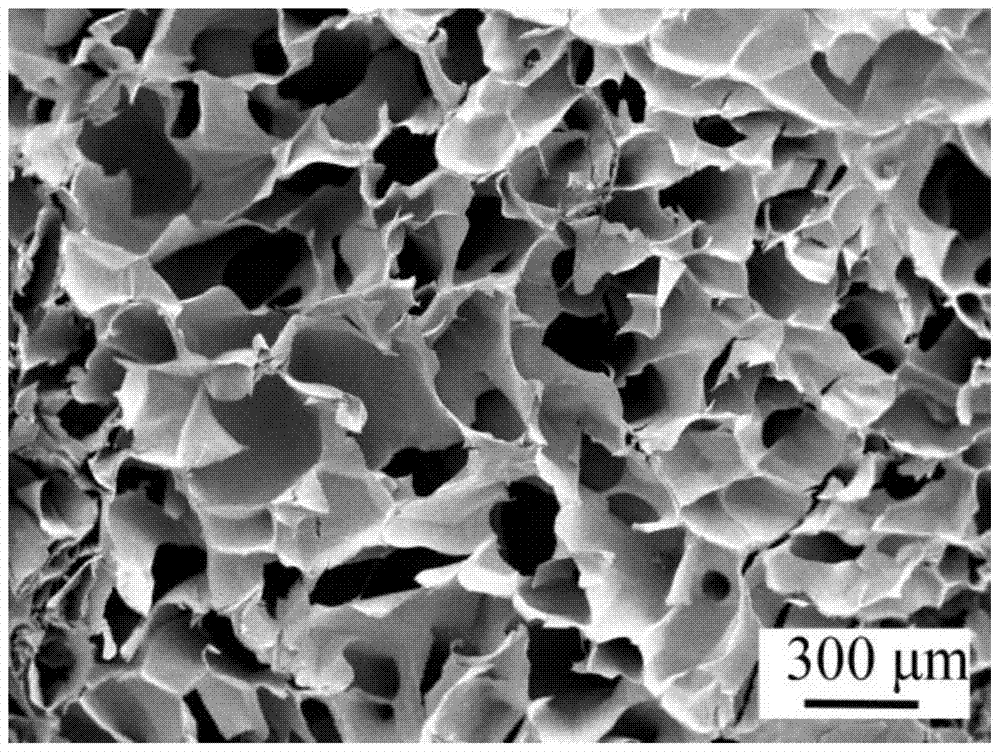
| pore size | aaaaa | aaaaa |
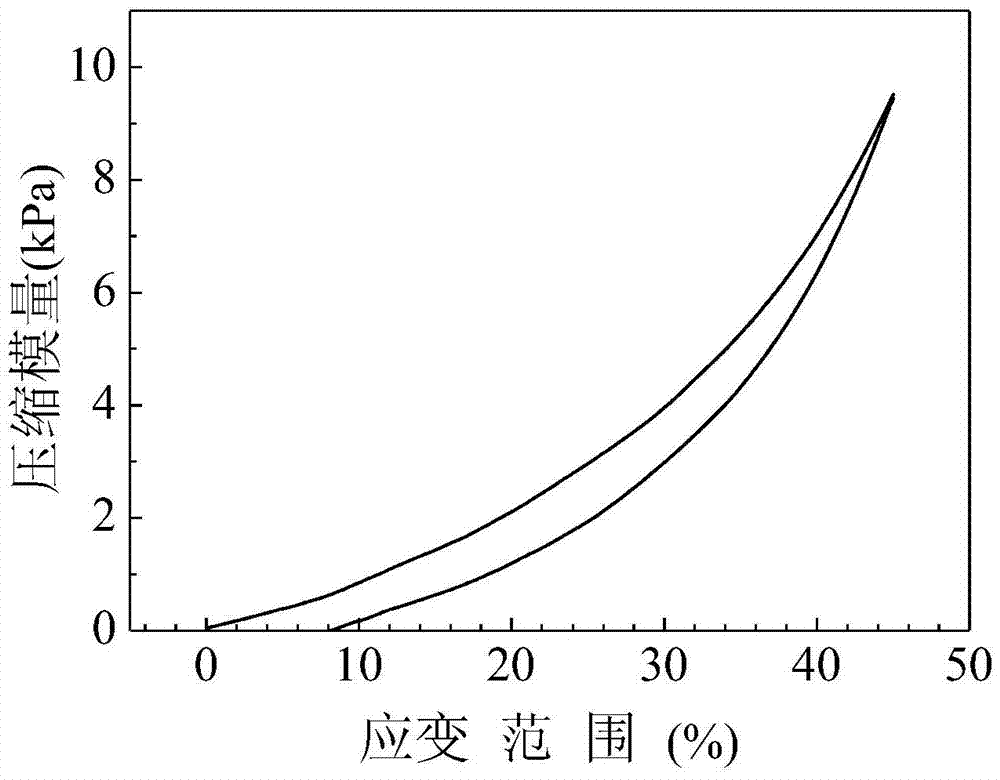
| compressive modulus | aaaaa | aaaaa |
| compressive modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com