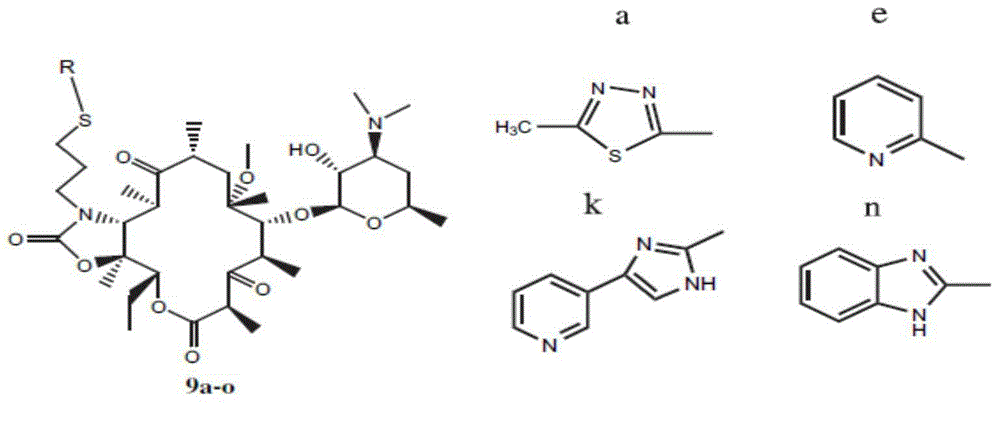
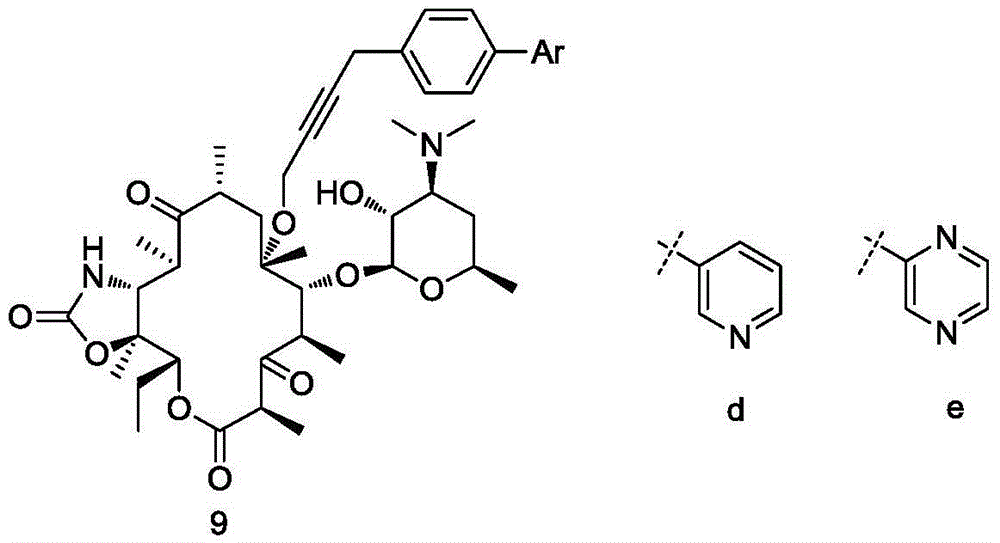
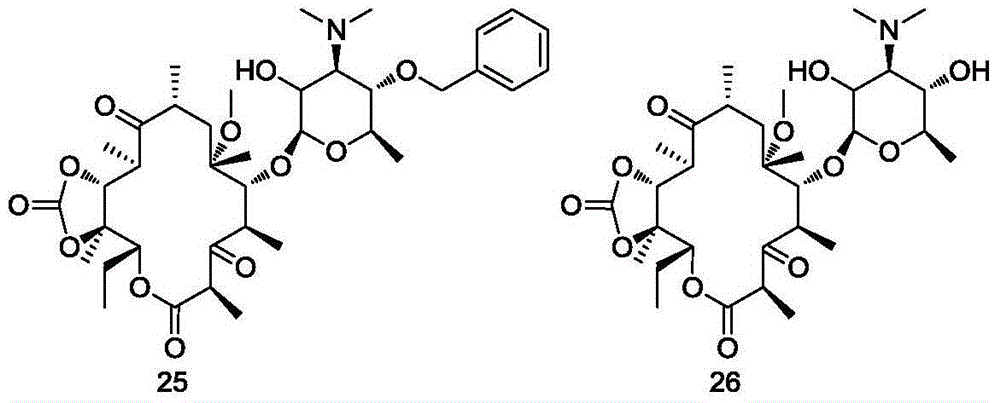
Erythromycin A ketolide antibiotic derivatives containing quinoline substituent group, and preparation methods and applications thereof
An erythromycin and substituent technology, which is applied in the field of erythromycin A ketolide antibiotic derivatives, can solve the problems of inability to improve antibacterial activity, inability to enhance macrolide and ribosomal subunits, etc., and achieve efficient synthesis and the effects of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0084] Preparation part
[0085] The structure of the compound was obtained by H NMR spectroscopy ( 1 HNMR), carbon nuclear magnetic resonance ( 13 CNMR) and mass spectrometry (MS) to determine. Proton and Carbon NMR shifts (δ) are given in parts per million (ppm). Proton nuclear magnetic resonance spectrum is measured with Mercury-300, Mercury-300 or Mercury-600 type nuclear magnetic resonance instrument, deuterated chloroform (CDCl 3 ) or heavy water (D 2 O) or deuterated dimethylsulfoxide (DMSO-d 6 ) as solvent and tetramethylsilane (TMS) as internal standard.
[0086] High-resolution mass spectrometry was determined by Agilent1100seriesLC / MSDtrapmassspectrometer or ThermoExactiveorbitrapplusLC / MSDmassspectrometer.
[0087] Column chromatography generally uses 160-200 mesh silica gel as the carrier.
[0088] Anhydrous solvents were all worked up by standard methods. Other reagents were commercially available analytically pure.
[0089] in,
[0090] Acacetyl
[00...
preparation example 1
[0125] Synthetic route of Scheme1 intermediate 5a
[0126]
[0127] Scheme 1 Reagents and conditions: a.CH 3 COSK, DIEA, Pd 2 (dba) 3 , Xantphos, 1,4-dioxane, macrowave; b.1), KOH, EtOH, 2), H + , r.t; c.K 2 CO 3 ,DMF,90℃; d.NH 2 NH 2 ·H 2 O, EtOH, reflux.
[0128] Synthesis of compound (2a)
[0129] 3-bromopyridine (1a) (250mg, 1.2mmol), potassium thioacetate (275mg, 2.4mmol), Pd 2 (dba) 3 (27.5mg, 0.03mmol), Xantphos (35mg, 0.06mmol) were added to a 10ml microwave tube, sealed with a three-way ventilation valve, vacuumed by a decompression pump, and the gas in the microwave tube was replaced three times with argon, and weighed with a syringe Inject the calculated amount of diisopropylethylamine (0.42ml, 2.4mmol) and 1,4-dioxane into the microwave tube, quickly remove the tee and quickly cover the mouth of the bottle with the microwave tube cap , placed in a microwave reactor, set the microwave reactor according to the following parameters (Power: 150W, Ramp: 1...
preparation example 2
[0144] Synthetic route of Scheme2 intermediate 3b
[0145]
[0146] Scheme 2 Reagents and conditions: a.N-(3-Bromopropyl)phthalimide, K 2 CO 3 ,DMF,90℃; b.NH 2 NH 2 ·H 2 O, EtOH, reflux, 80°C.
[0147] Synthesis of compound (2b)
[0148] 3-hydroxyquinoline (1b) (2.0g, 13.78mmol), N-(3-bromopropyl) phthalimide (3.7g, 13.78mmol), potassium carbonate (2.1g, 15.156mmol) They were added to N,N-dimethylformamide (40ml), heated to 90°C, and reacted for 10h. TLC (ethyl acetate / petroleum ether 1:3) showed complete reaction. Stop the reaction, cool the reaction solution to room temperature, filter to remove solids, concentrate the filtrate, dissolve the residue in dichloromethane, wash with water, wash with saturated brine, dry over anhydrous sodium sulfate, filter, and perform silica gel column chromatography (ethyl acetate / petroleum ether 1 :15) to obtain light yellow compound 2b (4.186g, yield 91.6%).
[0149]1 HNMR (400MHz, CDCl 3 ):δ8.457(s,1H),7.956(d,J=8Hz,1H),7.824~...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com