Method for simultaneously determining active peptide and free amino acid
A technology of free amino acids and active peptides, applied in the field of analysis and detection, can solve the problem of not being able to measure active peptides and free amino acids at the same time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
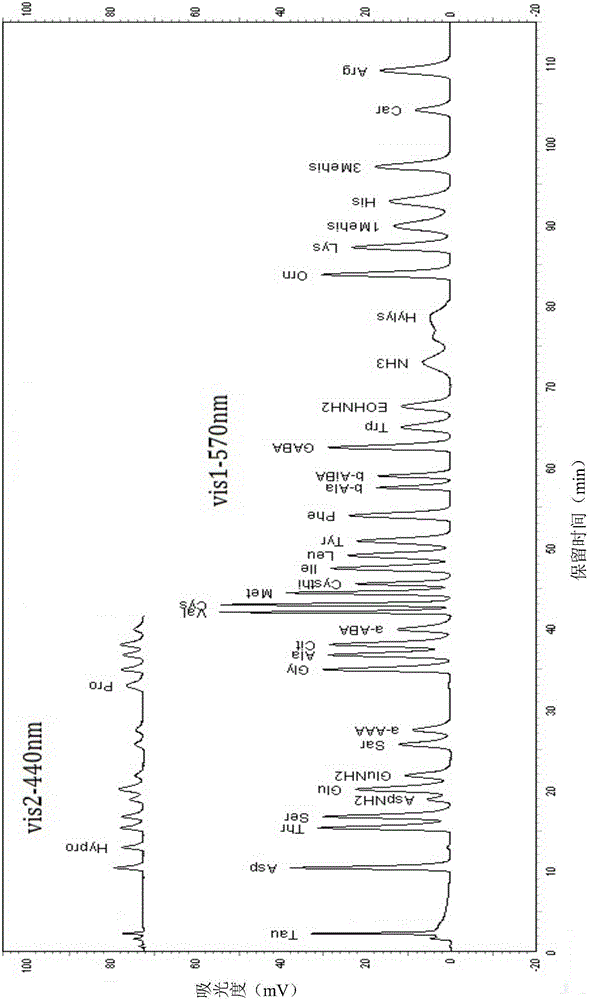
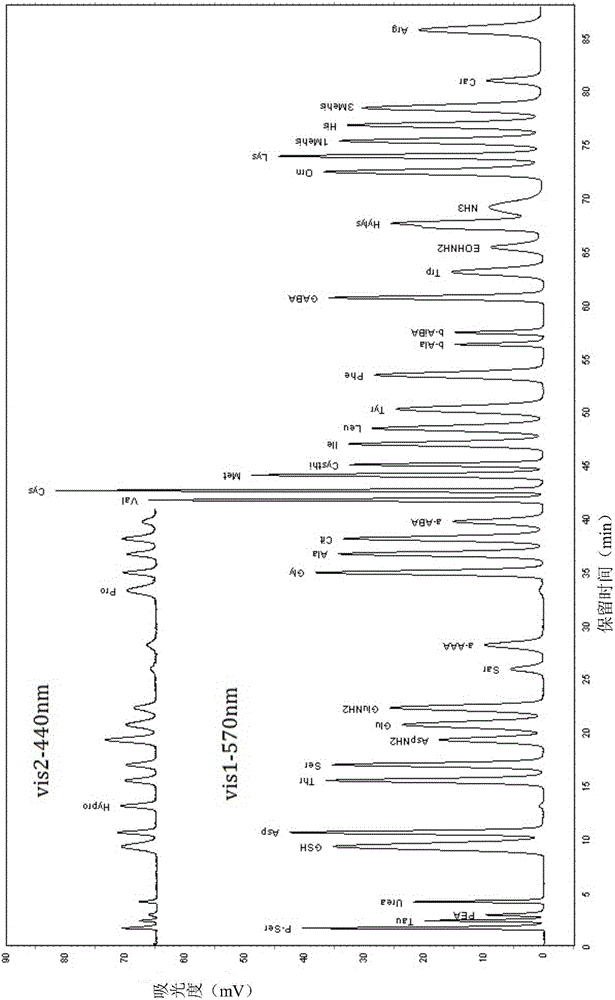
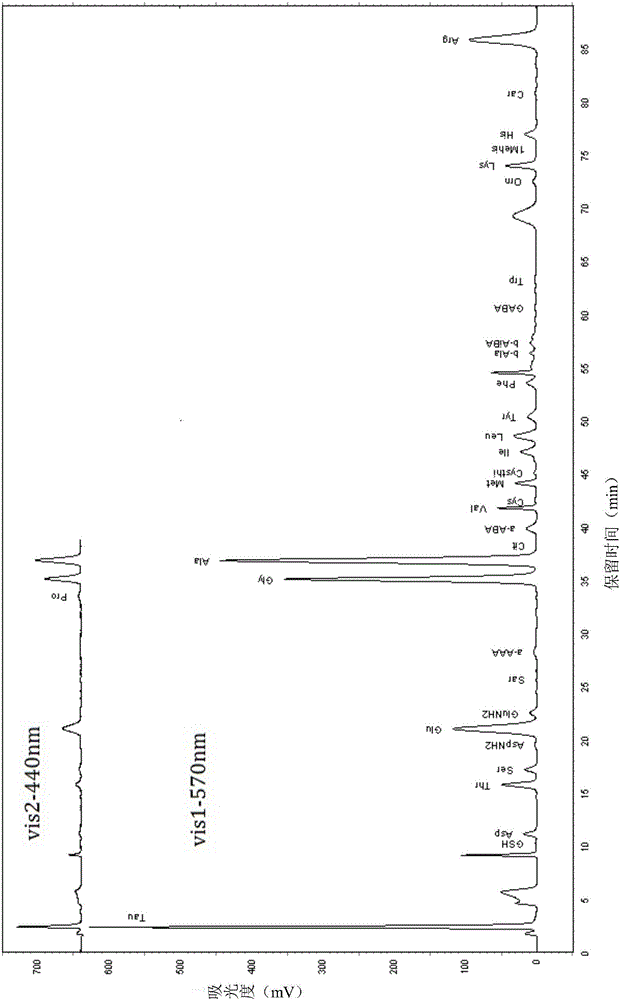
[0073] The analytical determination of embodiment 1 standard solution
[0074] (1) Preparation of elution buffer and ninhydrin derivative reagent
[0075] Prepare buffers B1-B5 according to Table 1; prepare ninhydrin derivatization reagents (ninhydrin reaction solution R1, reaction buffer R2, washing solution R3) according to Table 2.
[0076] Table 1 Composition of B1~B5 buffer
[0077]
[0078] The composition of table 2 ninhydrin derivative reagent
[0079]
[0080] (2) Amino acid and peptide standard solution preparation
[0081] Preparation of GSH (glutathione), Car (carnosine), P-Ser (phosphoserine), Tau (taurine), PEA (phosphoethanolamine), Urea (urea), Asp (aspartic acid), Hypro ( Hydroxyproline), Thr (threonine), Ser (serine), AspNH 2 (Asparagine), Glu (glutamic acid), GluNH2 (glutamine), Sar (sarcosine), a-AAA (α-aminocaproic acid), Pro (proline), Gly (glycine), Ala (alanine), Cit (citrulline), a-ABA (α-aminobutyric acid), Val (valine), Cys (cystine), Met ...
Embodiment 2
[0093] The determination experiment of embodiment 2 linear range and detection limit
[0094] Dilute the standard mixed solution by a certain factor with 0.02mol / L hydrochloric acid solution, and use the optimized elution program in Table 3 for three gradient elution tests for each concentration solution.
[0095] By the retention time of going out peak as qualitative basis, make standard curve with the peak area of measured chromatogram and its corresponding concentration, evaluate with linear correlation coefficient (R2); According to signal-to-noise ratio, when the peak of measured substance chromatographic peak When the height is 3 times of the noise (S / N=3), determine the minimum detection limit (LOD) of its mixed standard component; when the peak height of the chromatographic peak is 10 times of the noise (S / N=10), determine its mixed The limit of quantitation (LOQ) of a standard component is the lowest concentration value required to quantify the analyte.
[0096] Th...
Embodiment 3
[0101] The determination experiment of embodiment 3 recovery rate and precision
[0102] Take 2g shellfish sample tissue, add three mixed standard solutions with different concentrations of high, medium and low respectively to the sample, then add 15mL of 0.02mol / L dilute hydrochloric acid and 1mL of dithiothreitol with a concentration of 1wt%, and fully homogenize Ultrasonic cleaning was performed for 5 minutes, and then centrifuged with a refrigerated centrifuge (5000r, 4°C) for 10 minutes to collect the supernatant. Add 10 mL of dilute hydrochloric acid with a concentration of 0.02 mol / L to the remaining residue, stir, centrifuge again (5000 r, 4 °C) for 5 min, combine the supernatants, and dilute to 50 mL. After constant volume, pipette 2mL, add 2mL of 5wt% sulfosalicylic acid, centrifuge again (10000r, 4°C) for 10min, and then filter with a 0.22μm aqueous phase filter membrane to obtain the test solution.
[0103] Each concentration of the test solution was subjected to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com