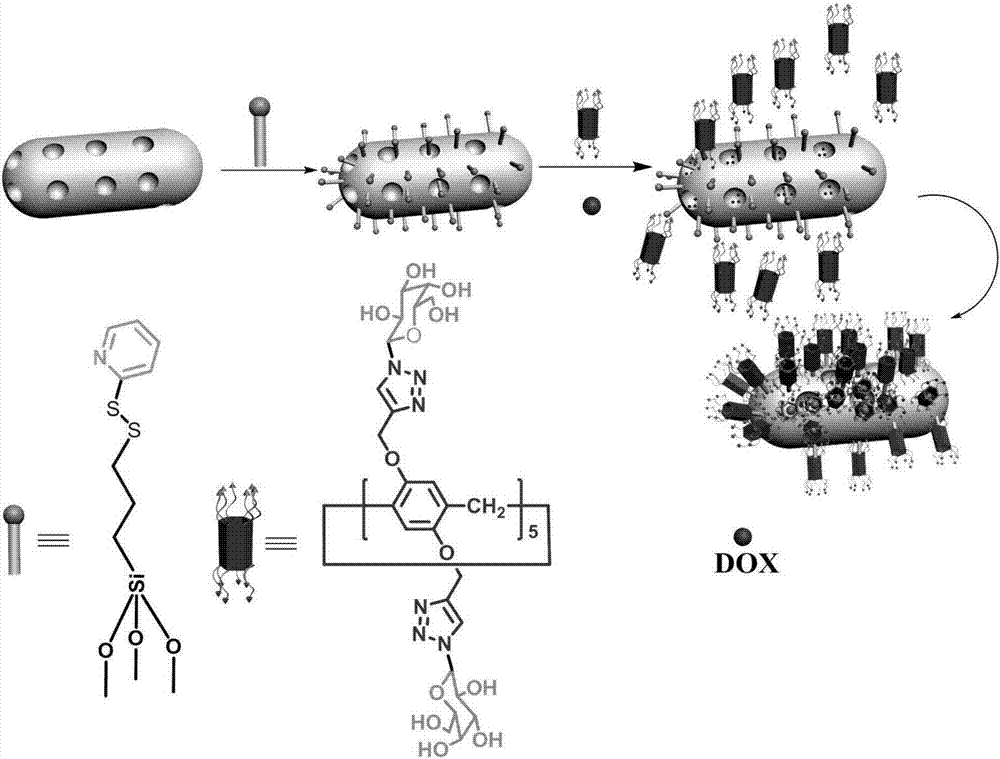
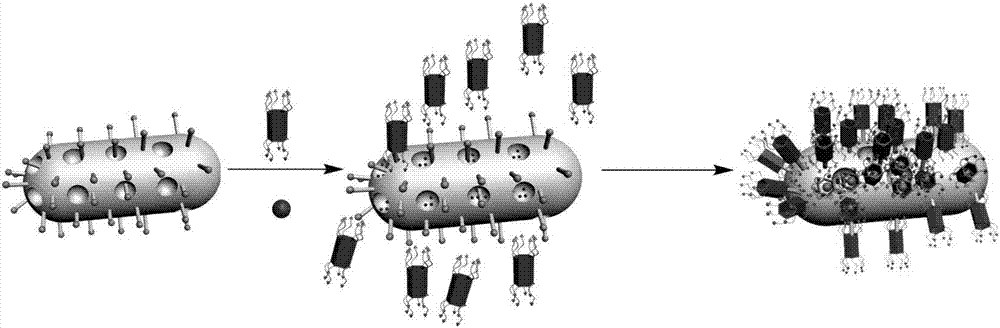
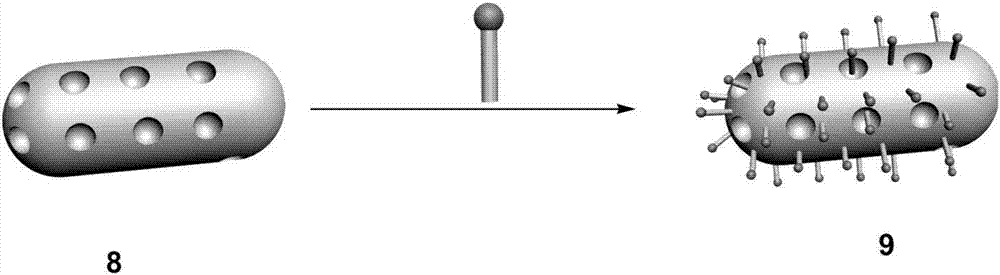
Multi-purpose cerium dioxide nanometer drug-loading system realizing targeted stimulation responsiveness
A stimuli-responsive, ceria-based technology, applied in the field of nano-biomedical materials, can solve problems affecting the toxicity of cancer cells, the limitation of synergy of cancer cells, etc., achieve good application and development prospects, and improve the effect of lethality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
[0045] Synthesis of galactose derivative 1: under nitrogen protection, 2g (5.12mmol) peracetylated galactose was dissolved in 15mL of dichloromethane; at the same time, 12mL of hydrobromic acid (concentration of hydrobromic acid 33%, dissolved In acetic acid, HBr) was obtained and reacted at room temperature for 2h. After the reaction of the raw materials was detected by TLC, 20 mL of ice water and 20 mL of dichloromethane were added to separate the layers. The aqueous layer was extracted with 3*20 mL of dichloromethane, and the organic layer was washed with 100 mL of saturated sodium bicarbonate until neutral. Finally, the organic layer was washed once with 50 mL of saturated sodium chloride, dried with anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain galactose derivative 1.
[0046] Since the galactose derivative 1 was very unstable, the next reaction was carried out directly. 2g galactose derivative 1, 1.6g (1eq.) tetrabutyl ...
Embodiment 2
[0048]
[0049] The synthesis of compound 4: with 11g hydroquinone 3 (100mmol), potassium carbonate (K 2 CO 3 ) 27.6g (200mmol), 47.5g (200mmol) of propyne bromide were dissolved in 180mL of acetonitrile, and heated to reflux for 24h under nitrogen protection. After the TCL detection reaction is completed, filter and spin dry in vacuum, dissolve with dichloromethane and wash with distilled water three times. After liquid separation, the organic layer was dried with anhydrous sodium sulfate and separated by column chromatography to obtain compound 4 (89%).
Embodiment 3
[0051]
[0052] Synthesis of Compound 5: Dissolve 2.72g of Compound 4 (20mmol) in 60mL of dichloroethane, add 1.398g of paraformaldehyde (40mmol) under nitrogen atmosphere, and stir for 1 hour at room temperature with 4ml of boron trifluoride diethyl ether. After the reaction was detected by TLC, ice water was added to quench, the organic layer was washed three times with water and saturated sodium chloride three times, and finally dried with anhydrous sodium sulfate, and separated by column chromatography to obtain compound 5 (75%).
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com