Novel lysine decarboxylase mutant and application thereof
A technology of lysine decarboxylase and amino acid is applied in the application field of producing 1,5-pentanediamine, which can solve the problems of limited application value and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Construction of embodiment 1.CadA wild-type bacterial strain
[0084] E.coli MG1655 (obtained from ATCC 700926, can refer to Blattner FR et al., The completegenome sequence of Escherichia coli K-12.Science 277:1453-62 (1997)) in LB medium (tryptone 10g / L, yeast powder 5g / L, sodium chloride 10g / L, pH 7.0), 37°C, 200rpm, after culturing for 12-16h, the cells were collected, and the genomic DNA was extracted using the Biomiga genome mini-prep kit. Using the Escherichia coli genome as a template, with cadA-F (as shown in SEQ ID NO: 3) and cadA-R (as shown in SEQ ID NO: 4) as primers, amplify the cadA gene (sequence as shown in SEQ ID NO: 2 shown), cloned into the vector pET-21a(+) (purchased from NOVAGEN) by 5'NdeI and 3'XhoI to obtain the pET21-cadA expression plasmid, and then transformed it into E.coli BL21(DE3) to obtain E.coli BL21(DE3). coli BL21(DE3) / pET21-cadA wild type engineering strain.
Embodiment 2
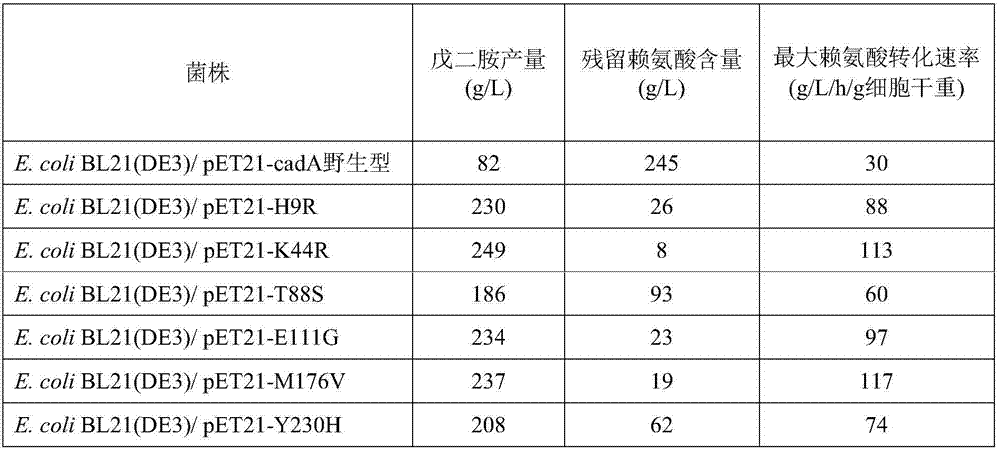
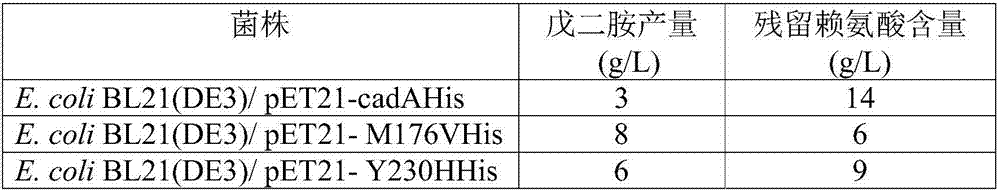
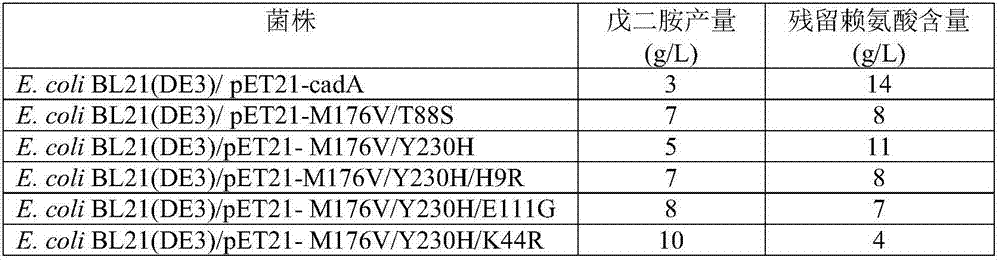
[0085] The acquisition of embodiment 2.CadA mutant strain
[0086] Utilize the Stratagene Series XL-II site-directed mutagenesis kit, respectively designed 6 pairs of primers (see Table 1), using the constructed pET21-cadA wild-type plasmid as a template, respectively used 6 pairs of primers for PCR amplification, respectively, the 9th amino acid of CadA Histidine is mutated to arginine, lysine at 44 is mutated to arginine, threonine at 88 is mutated to serine, glutamic acid at 111 is mutated to glycine, methionine at 176 is mutated to valine , the tyrosine at position 230 was mutated to histidine, and the resulting plasmids expressing the mutants were named pET21-H9R, pET21-K44R, pET21-T88S, pET21-E111G, pET21-M176V and pET21-Y230H, respectively. The PCR reaction conditions were: 95° C. for 5 min, 25 cycles (95° C. for 30 s, 50° C. for 30 s, 68° C. for 9 min), and 68° C. for 10 min. PCR amplification system (50 μL): template 1 μL, upstream and downstream primers 2 μL, dNTP ...
Embodiment 3
[0089] The production of pentamethylenediamine of embodiment 3.CadA wild type and mutant engineering strain
[0090] The thalline culture of engineering strain and the expression of lysine decarboxylase: the bacterial lawn of the appropriate plate activation of each engineering bacterium is inoculated in 100ml liquid LB culture medium (peptone 1%, yeast powder 0.5%, sodium chloride 1% , pH 7.0) in the 500ml seed bottle, add 0.1g / L ampicillin, shake culture at 37 ℃, 220rpm for 12 hours; , ammonium chloride 8g / L, potassium dihydrogen phosphate 5g / L, magnesium sulfate heptahydrate 0.5g / L, ferrous sulfate heptahydrate 0.2g / L) in the 5L fermentation tank, add 0.1g / L ampicillin, ferment The temperature is 37°C, the stirring speed is 300rpm-900rpm, the air flow rate is 1-4vvm, the ammonia solution is used to control the pH to 7.0 during the fermentation process, the added glucose is controlled at about 10g / L, and the OD 600 When growing to 30, add 0.1mM IPTG to induce the expression...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com