Nano cellulose containing cinnamyl functional group and preparation method and application of nano cellulose
A nano-cellulose and functional-based technology, which can be used in skin care preparations, pharmaceutical formulations, cosmetic preparations, etc., can solve the problems of mixing nano-cellulose and hydrophobic matrix materials, and achieve excellent UV absorption performance, moisturizing The effect of increasing wetness and improving dispersibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
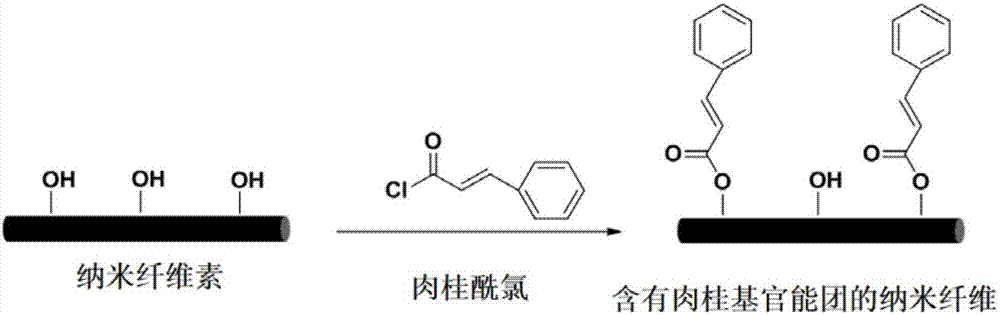
[0062] The schematic diagram of introducing cinnamyl functional groups on cellulose nanocrystals by acylation method is as follows figure 1 shown.
[0063] 1 gram of nanocellulose is dispersed into water by ultrasonic method, and the mass fraction of nanocellulose is 1% dispersion liquid A; in dispersion liquid A, add 1 gram of 4-dimethylaminopyridine, 1 gram of triethylamine and 0.5 g of cinnamoyl chloride was used to obtain a mixed solution B; the above mixed solution B was reacted at 25° C. for 24 hours, washed with acetone for 3 times, and nanocellulose containing cinnamyl functional groups could be obtained.
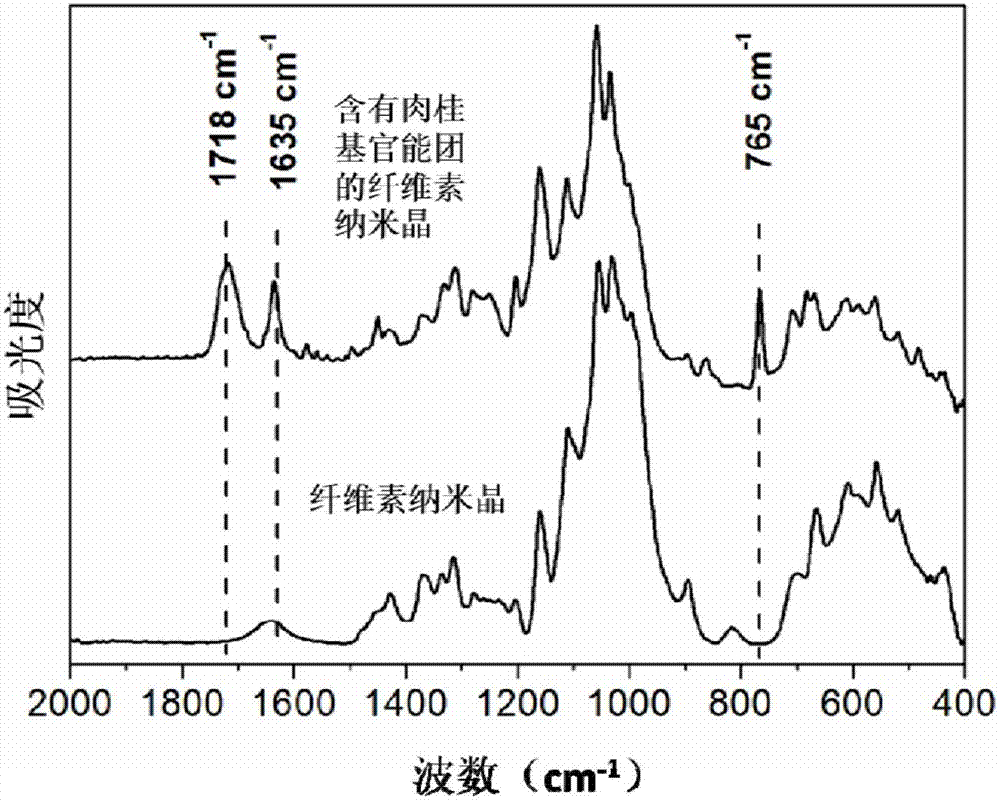
[0064] The Fourier transform infrared spectrum of the cellulose nanocrystals containing cinnamyl functional groups prepared by this method is as follows: figure 2 As shown, compared with nanocellulose, the characteristic peak of cinnamyl functional group appeared in the infrared spectrum of nanocellulose containing cinnamyl functional group, such as at 1718cm -1 ...
Embodiment 2
[0068] The steps of introducing cinnamyl functional groups on cellulose nanocrystals by esterification are as follows:
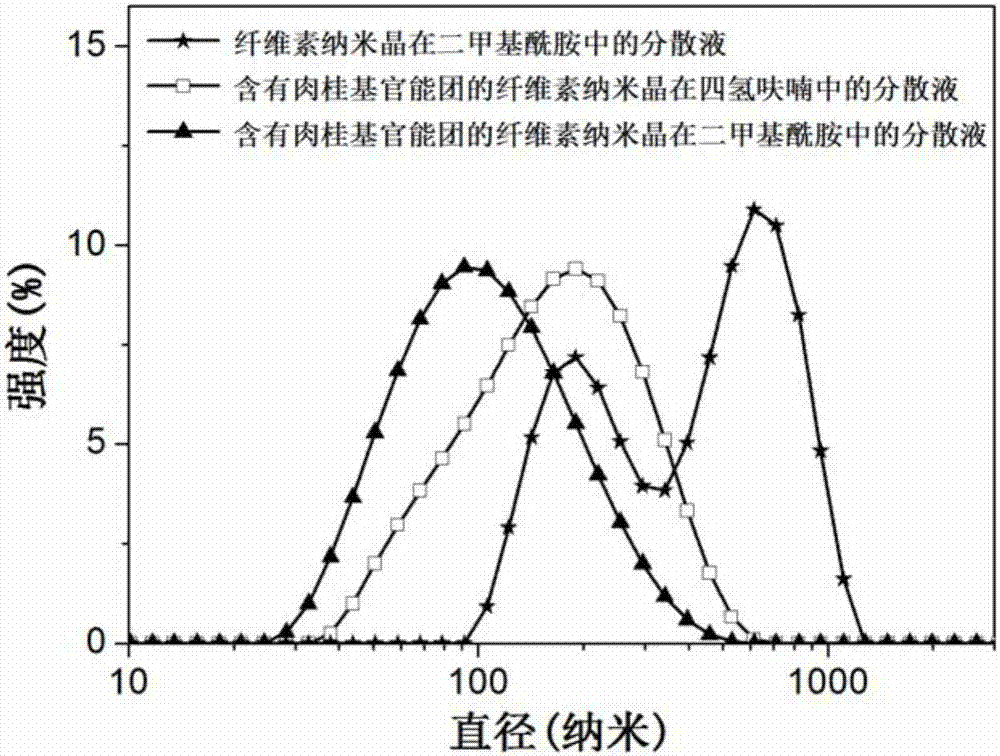
[0069] Disperse 1.3 g of cellulose nanocrystals into 100 ml of water, add 20 mg of 2,2,6,6-tetramethylpiperidine-1-oxide and 400 mg of sodium bromide, stir for 30 minutes, add sodium hydroxide solution Regulate the pH value of reaction solution to be 10, then add 8 grams of mass fraction and be 13% sodium hypochlorite solution; Continue to add sodium hydroxide in reaction solution to keep the pH of reaction solution at more than 9, when the pH of reaction solution is basically stable For this purpose, at the end of the TEMPO oxidation reaction, add 10 ml of methanol, then adjust the pH of the reaction solution to be neutral with hydrochloric acid, and obtain cellulose nanocrystals oxidized by TEMPO after dialysis for 7 days; cellulose nanocrystals oxidized by TEMPO and cellulose nanocrystals oxidized by TEMPO Infrared spectrum such as Figure 5 As shown, co...
Embodiment 3
[0072] The steps of introducing polyhydroxycinnamic acid methacrylate on the nanocellulose by in situ initiating free radical polymerization on the nanocellulose are as follows:
[0073] Firstly, the monomer hydroxycinnamate methacrylate is prepared by the acylation reaction of hydroxyethylmethacrylate (2-Hydroxyethylmethacrylate, abbreviated as HEMA) and cinnamoyl chloride.
[0074] 1 g of hydroxyethyl methacrylate and 2 g of cinnamoyl chloride were reacted in a mixture of 100 ml of dichloromethane and 20 ml of triethylamine at room temperature for 24 hours.
[0075] The reaction product is purified by silica gel chromatography to finally obtain the monomer hydroxycinnamate methacrylate (CEM). CEM in deuterated chloroform1 The H NMR spectrum is: 1 H NMR (400MHz, CDCl3): δ1.89 (3H, t, CH3); 4.37 (4H, m, -O-CH2-CH2-); 5.53 (1H, quin, =CH), 6.08 (1H, quin, =CH), 6.39(1H, d, =CH-Ar), 7.64(1H, d, =CH-CO-), 7.3~7.5(5H, m, ArH), such as Figure 7 shown.
[0076] Disperse 1 gram ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydrodynamic diameter | aaaaa | aaaaa |
| Hydrodynamic diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com