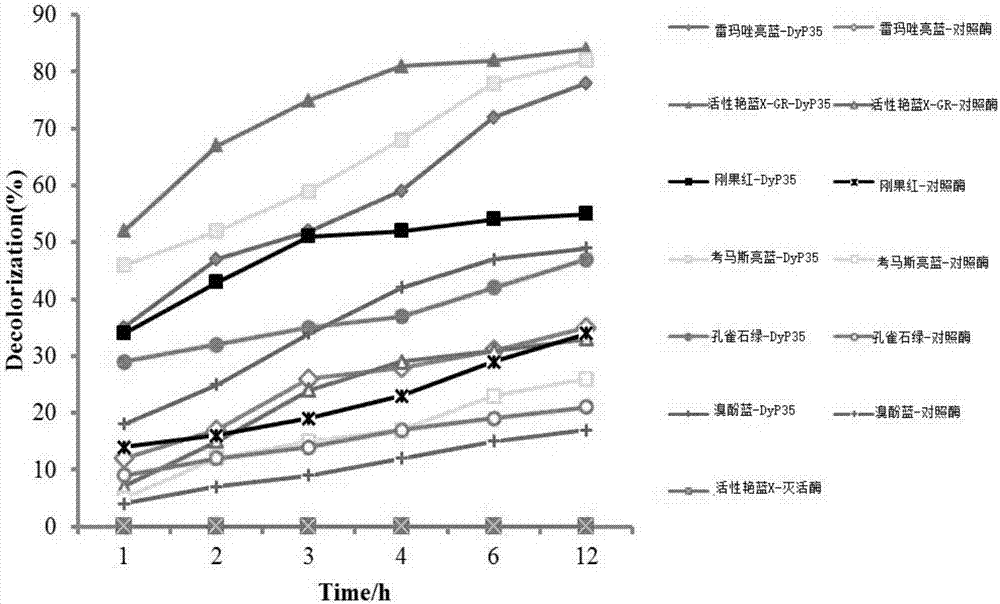
Peroxidase DyP35 gene, and expression protein and application thereof
A peroxidase and gene technology, applied in the field of bioengineering, can solve the problems of low enzyme activity, poor cost, unfavorable effect, etc., and achieve the effect of high enzyme activity and high decolorization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
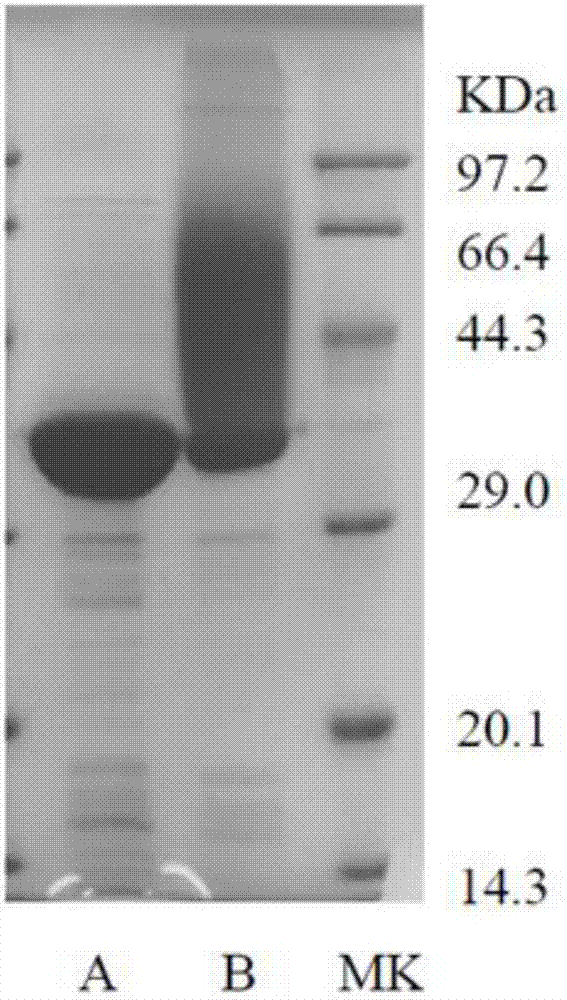
[0026] Embodiment 1: the purification of protein
[0027] (1) Ammonium sulfate precipitation: Cultivate Comamonas serinivorans SP35 in LB medium (with 1 g / L lignin added as an inducer) for 24 hours, then centrifuge at 12000 rpm to collect the cells, and wash with buffer (Tris- Hydrochloric acid, pH 8.0) was washed once and re-centrifuged, then placed on ice for ultrasonic crushing, 15000 rpm high-speed centrifugation for 20 min, the supernatant was extracted and stirred on a magnetic stirrer, and ammonium sulfate was added in sections and then centrifuged to obtain proteins of different stages Precipitate, then detect the activity, collect the protein in the highly active ammonium sulfate precipitation concentration stage, and then dialyze to remove the ammonium sulfate.
[0028] (2) Ion-exchange column chromatography: The protein solution obtained by dialysis was purified by DEAE-SepharoseCL-6B chromatography column, and linearly eluted with 50 mmol / L Tris-HCl of five column ...
Embodiment 2
[0029] Embodiment 2: Determination of amino acid sequence
[0030] The single protein band obtained in Example 1 was subjected to N-terminal sequencing by Edman degradation method (Suzhou Hongxun Biotechnology Co., Ltd.), and the amino acid sequence of the decolorizing enzyme was obtained, as shown in SEQ ID NO.2. The sequence was compared and analyzed in the GENEBANK database, and the protein was determined to belong to the peroxidase class, named DyP35. At the same time, the nucleotide sequence of the enzyme gene was obtained, as shown in SEQ ID NO.1.
Embodiment 3
[0031] Embodiment 3: the construction of the engineering bacterium of dye decolorization peroxidase DyP35
[0032] (1) Synthesis of dye decolorization peroxidase DyP35 gene
[0033] The full-length gene of dye decolorization peroxidase DyP35 was synthesized by in vitro total gene synthesis method (synthesized by Suzhou Hongxun Biotechnology Co., Ltd.).
[0034] (2) Treatment and connection of dye decolorization peroxidase DyP35 gene
[0035] dye decolorization peroxidase DyP35 gene fragment with T 4 After DNA Polymerase treatment, link with plasmid pET-28a(+) (Suzhou Hongxun Biotechnology Co., Ltd.) DNA at room temperature for 20 min.
[0036] (3) Transformation of recombinant plasmids in Escherichia coli BL21
[0037] Add the ligation product to 50 µL E. coliArctic Expression (DE3) competent cells were placed in an ice bath for 30 min, then heat-shocked for 60 s and continued to ice-bath for 2 min, then added 250 µL of LB medium, and incubated at 37°C for 1 h. Then take...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com