Lead-containing raw material wet recovery treatment method
A technology of recovery treatment and treatment method, which is applied in the field of wet recovery of lead-containing raw materials, can solve the problems of lack of impurity removal process, etc., and achieve the effects of controllable particle size, high lead content and controllable shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
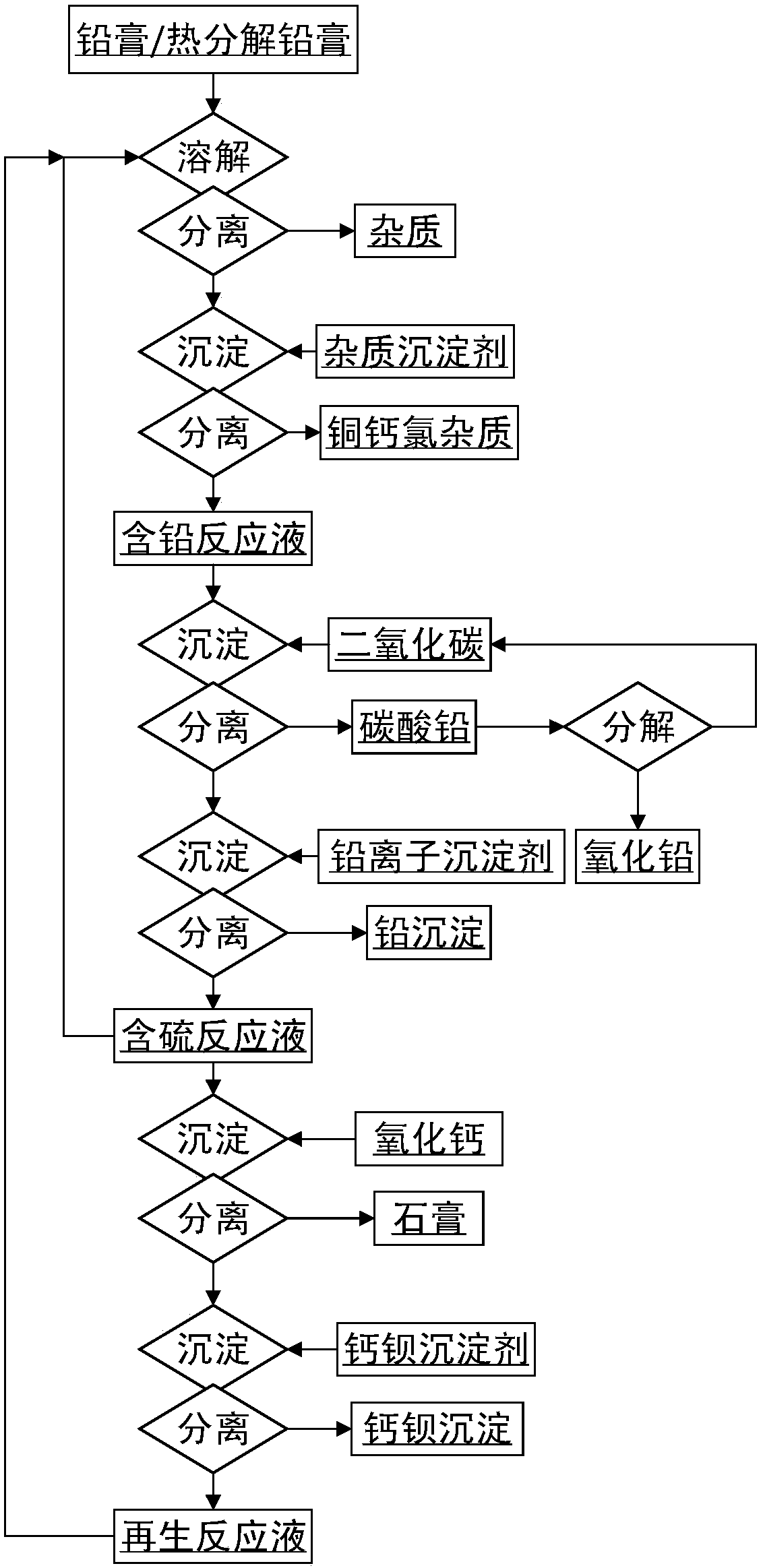
[0077] 1) Configure 50L containing 1.0mol / L ethylenediamine, 0.5mol / L diethylenetriamine, 1.0mol / L aspartic acid, 1.5mol / L sodium sulfate, 1g / L sodium lauryl sulfate, 0.1g / L xylenol orange reaction solution, add 5 kg of thermally decomposed lead plaster at 60°C, use sodium hydroxide to maintain the pH value of the solution between 9.0-9.5, react for 1.5 hours, and filter to obtain a lead-containing reaction solution;
[0078] 2) Add 1 g of sodium silicate to the lead-containing reaction solution obtained in step 1), filter to obtain lead-containing reaction solution B and impurity precipitation;
[0079] 3) Infuse carbon dioxide into the lead-containing reaction solution B obtained in step 2), the ventilation rate is 50L / min, the reaction temperature is controlled between 95-100°C, and sodium hydroxide is used to maintain the pH value of the solution between 8.0-8.5 during this period , the reaction time was 8 hours, filtered and washed to obtain the product lead carbonate an...
Embodiment 2
[0084] 1) Prepare 5L of reaction solution containing 0.8mol / L alanine, 1.2mol / L histidine, 1mol / L ammonium sulfate, 0.01mol / L cobalt sulfate, 1.1mol / L ethanolamine, and add lead paste at 80°C 0.8kg, 105g of 30% hydrogen peroxide, use ammonia water to maintain the pH value of the solution between 8.0-8.5, react for 2.5 hours, filter to obtain the lead-containing reaction solution;
[0085] 2) Add 1 g of ammonium oxalate and 2 g of ammonium phosphate to the lead-containing reaction solution obtained in step 1), filter to obtain lead-containing reaction solution B and impurity precipitation;
[0086] 3) Pass carbon dioxide into the lead-containing reaction solution B obtained in step 2), the ventilation rate is 10L / min, the reaction temperature is controlled between 80-85°C, and ammonia water is used to maintain the pH value of the solution between 7.0-7.5 during the reaction. The time is 4 hours, filtered and washed to obtain the product lead carbonate and sulfur-containing reac...
Embodiment 3
[0092] 1) Prepare 500L of reaction solution containing 0.2mol / L phenylalanine, 1.2mol / L lysine, 2.0mol / L potassium sulfate, 2g / L sodium pyrophosphate, add 100kg of thermally decomposed lead paste at 70°C, Use potassium hydroxide to maintain the pH value of the solution between 8.5-9.0, react for 3.5 hours, and filter to obtain a lead-containing reaction solution;
[0093] 2) Add 15 g of tannic acid and 200 g of potassium phosphate to the lead-containing reaction solution obtained in step 1), filter to obtain lead-containing reaction solution B and impurity precipitation;
[0094]3) Add 1kg of commercially available lead carbonate to the lead-containing reaction solution B obtained in step 2), and pass in carbon dioxide, the ventilation rate is 70L / min, and the reaction temperature is controlled between 70-75°C, during which potassium hydroxide is used to maintain the solution The pH value is between 6.0-6.5, the reaction time is 5 hours, filtered and washed to obtain the produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com