Nitroso grafted carbonate electrolyte of lithium battery and preparation method
A nitroso, electrolyte technology, applied in the manufacture of electrolyte batteries, secondary batteries, non-aqueous electrolyte batteries, etc., can solve the problems of unsolved dielectric constant and viscosity of electrolyte solvents, improve antioxidant performance, reduce Binding energy, overall viscosity reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
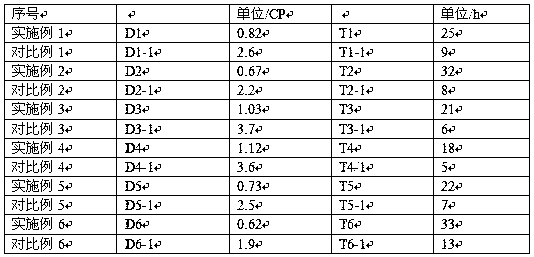
Image
Examples
Embodiment 1
[0027] A nitroso-grafted carbonate electrolyte for a lithium battery and a preparation method thereof, wherein lithium nitrite is mixed with ethylene carbonate and propylene carbonate, wherein the mixing ratio of ethylene carbonate and propylene carbonate is 1:0.5, Add fluoroethers and borates as additives simultaneously, the ratio of fluoroethers to borates is 1:0.3 to form an electrolyte, and the molar fraction of lithium nitrite in the electrolyte formed by this mixed solution is 0.435 is NO 2 - The mole fraction of ethylene carbonate is 0.435, the mole fraction of ethylene carbonate is 0.29, and the mole fraction of propylene carbonate is 0.145; that is, the mole fraction of ethylene carbonate is + the mole fraction of propylene carbonate is 0.435, while the mole fraction of alkenyl and carbonyl The sum of the scores is 0.87, which is NO 2 - The ratio of the mole fraction of the mole fraction to the sum of the mole fractions of alkenyl and carbonyl groups is 1:2; the mo...
Embodiment 2
[0029] A kind of nitroso grafted carbonate electrolyte of lithium battery and preparation method thereof, lithium nitrite salt is mixed with ethylene carbonate and propylene carbonate, wherein the mixing ratio of ethylene carbonate and propylene carbonate is 1:1, Add fluoroethers and borates as additives simultaneously, the ratio of fluoroethers to borates is 1:0.3 to form an electrolyte, and the molar fraction of lithium nitrite in the electrolyte formed by this mixed solution is 0.493 is NO 2 - The mole fraction of ethylene carbonate is 0.493, the mole fraction of ethylene carbonate is 0.125, and the mole fraction of propylene carbonate is 0.125; that is, the mole fraction of ethylene carbonate is + the mole fraction of propylene carbonate is 0.25, while the mole fraction of alkenyl and carbonyl The sum of the scores is 0.5, which is NO 2 - The ratio of the mole fraction of the mole fraction to the sum of the mole fractions of alkenyl and carbonyl groups is 1:1; the mole ...
Embodiment 3
[0031] A nitroso-grafted carbonate electrolyte for a lithium battery and a preparation method thereof. Lithium nitrite is mixed with ethylene carbonate, and fluoroethers and borates are added as auxiliary agents, and fluoroethers and boron The ratio of acid salt is 1:0.3 to form an electrolyte, and the mole fraction of lithium nitrite in the electrolyte formed by the mixed solution is 0.435, that is, NO 2 - The mole fraction of ethylene carbonate is 0.435, and the mole fraction of ethylene carbonate is 0.435; that is, the mole fraction of ethylene carbonate + propylene carbonate is 0.435, and the sum of the mole fractions of alkenyl and carbonyl is 0.87, that is, NO 2 - The ratio of the mole fraction of the mole fraction to the sum of the mole fractions of alkenyl and carbonyl groups is 1:2; the mole fraction of fluoroethers is 0.1, and the mole fraction of borates is 0.03; it is heated in a water bath at a low temperature of 40°C and Stir quickly; adjust the pH value to 7.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com