Dynamic polymer and application thereof
A polymer, dynamic technology, applied in the field of intelligent polymer materials, can solve the problems of difficult to fully reflect dynamic covalent bonds, difficult characteristics, difficult controllability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
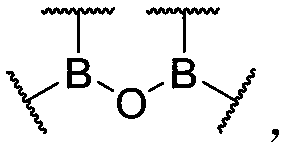
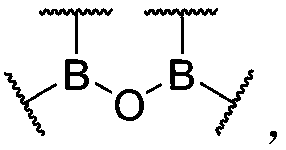
[0086] According to an embodiment of the present invention, the organoboroxine-boron bond has the following structure:
[0087]
[0088] Wherein, each boron atom in the structure is connected to at least one carbon atom through a boron-carbon bond, and at least one organic group is connected to a boron atom through the boron-carbon bond; each boron atom in the structure can form One or two organoboroxine bonds, different organoboroxine bonds can be connected to form a ring or not connected to form a ring; Indicates a connection to any suitable atom, group, substituent, polymer chain, at least two different boron atoms via at least one on each Incorporated into the polymer chain; different on the same boron atom It can be connected to form a ring or not to form a ring, and the boron atoms on different boron atoms The rings may also be connected to form a ring or not connected to form a ring, and the rings include but not limited to aliphatic rings, aromatic rings, ethe...
Embodiment 1
[0228] Starting from ethylene, 4-vinylphenylboronic acid, and neopentyl glycol 4-vinylphenylboronic acid, the reaction was carried out in a flask connected to a mineral oil bubbler. A mixed gas of nitrogen and ethylene was introduced to keep the pressure of ethylene in the reaction system at 0.1 atm. Add 4-vinylphenylboronic acid (0.05M) in chlorobenzene / toluene solution, 4-vinylphenylboronic acid neopentyl glycol ester (0.04M) in chlorobenzene / toluene solution, Pd-α-diimine catalyst chlorine Benzene / toluene solution was stirred at room temperature for 26 h, and then excess triethylsilane was added under nitrogen atmosphere to terminate the polymerization reaction. Separation, concentration, and drying are carried out at last to obtain pure dendritic polyethylene containing polyphenylboronic acid (ester) end groups (101 branches per 1000CH 2 , molecular weight reaches 45000), wherein the total insertion rate of 4-vinylphenylboronic acid and 4-vinylphenylboronic acid ester is ...
Embodiment 2
[0232] Using equimolar amounts of 1-amino-5-hexene and propyl isocyanate as raw materials, react at room temperature under a nitrogen atmosphere to prepare ureido-containing olefins. Add 85ml of toluene, 0.8ml of ureido-containing olefin, 1.8ml of but-3-ene ethylene glycol borate, and 10ml of MAO to the reaction flask rinsed with the toluene solution of triisobutylaluminum. After the reaction system is thermally balanced, add ethylene until The ethylene pressure in the system is 2 bar. Then add 2ml rac-Me 2 Si(2-MeBenz[e]Ind) 2 ZrCl 2 (1μmol) toluene solution, the polymerization starts, and the ethylene pressure is kept constant during the reaction. After reacting for 10 min, methanol was added to terminate the reaction. Subsequently, methanol precipitation, hydrochloric acid aqueous solution treatment, filtration, deionized water treatment, and vacuum drying at 70° C. obtained a propylene copolymer containing boric acid and ureido side groups.
[0233] Take 35g of propyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| elastic modulus | aaaaa | aaaaa |
| elastic modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com