Synthesis method and application of unsaturated hyperbranched polyimide resin
A technology of polyimide resin and synthesis method, applied in coating and other directions, can solve the problems of difficulty in reducing the amount of diluent, high molecular weight of polyimide, uncontrollable degree of branching, etc., and achieves excellent processing and molding performance, high Solubility, avoid gelation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
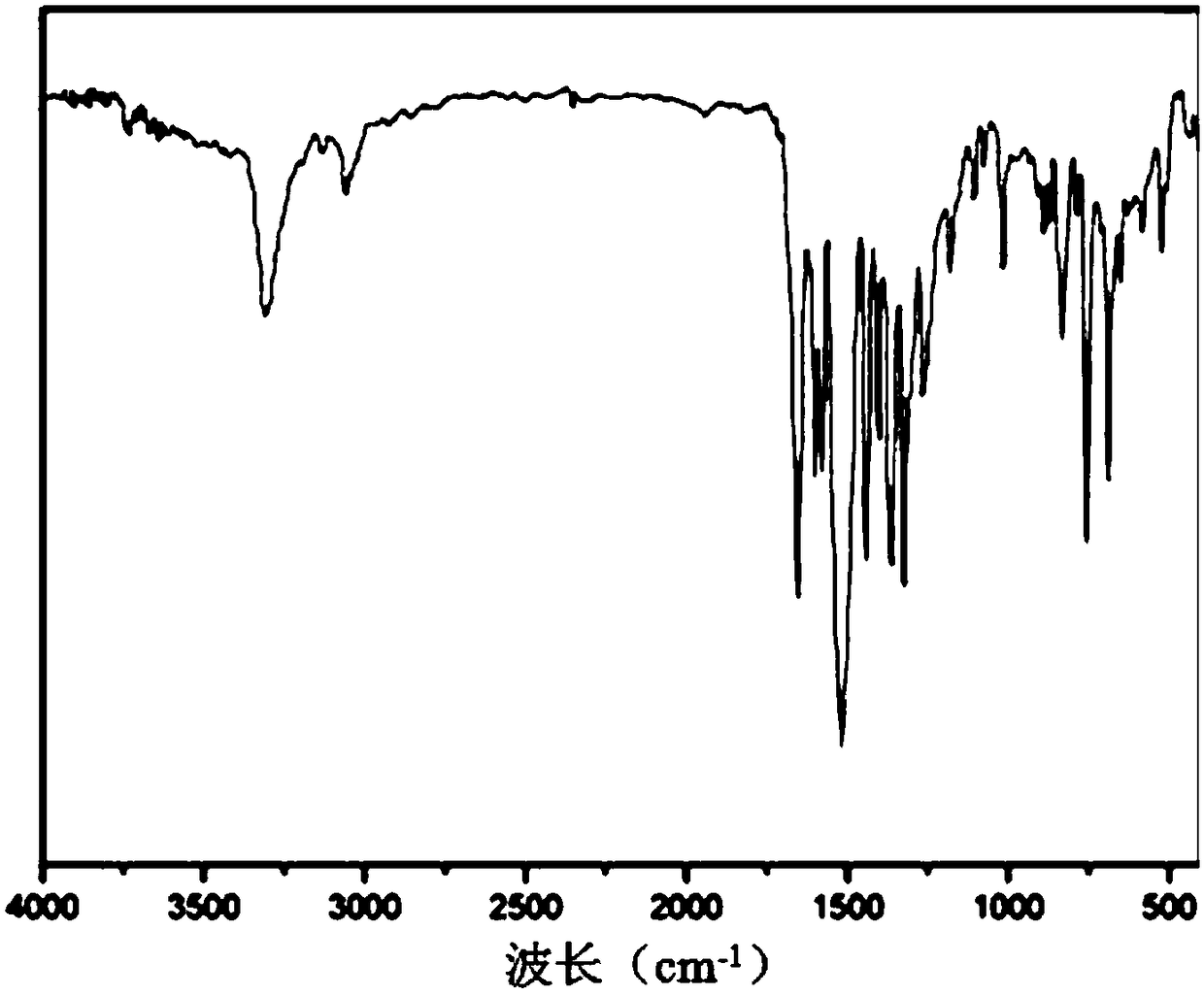
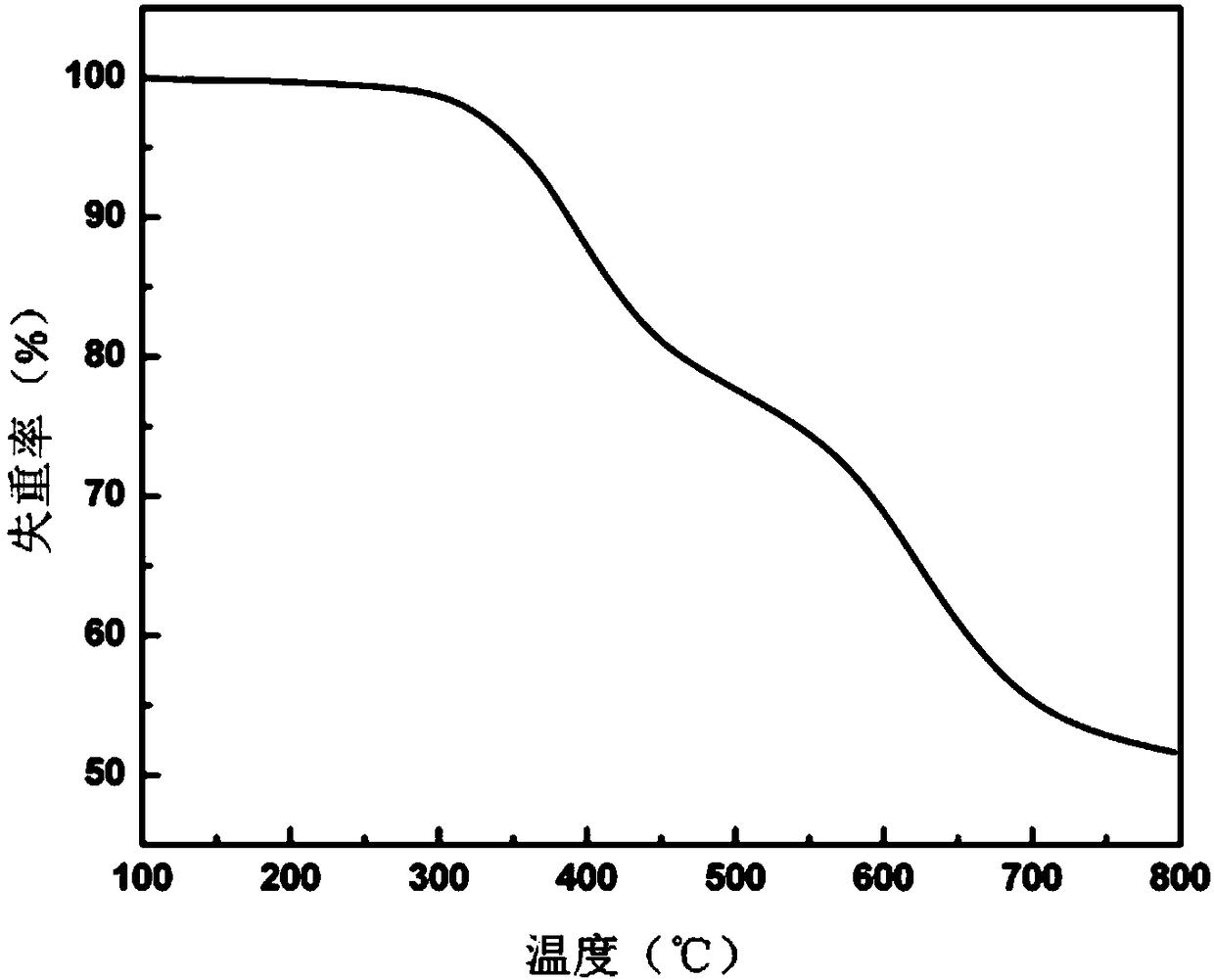
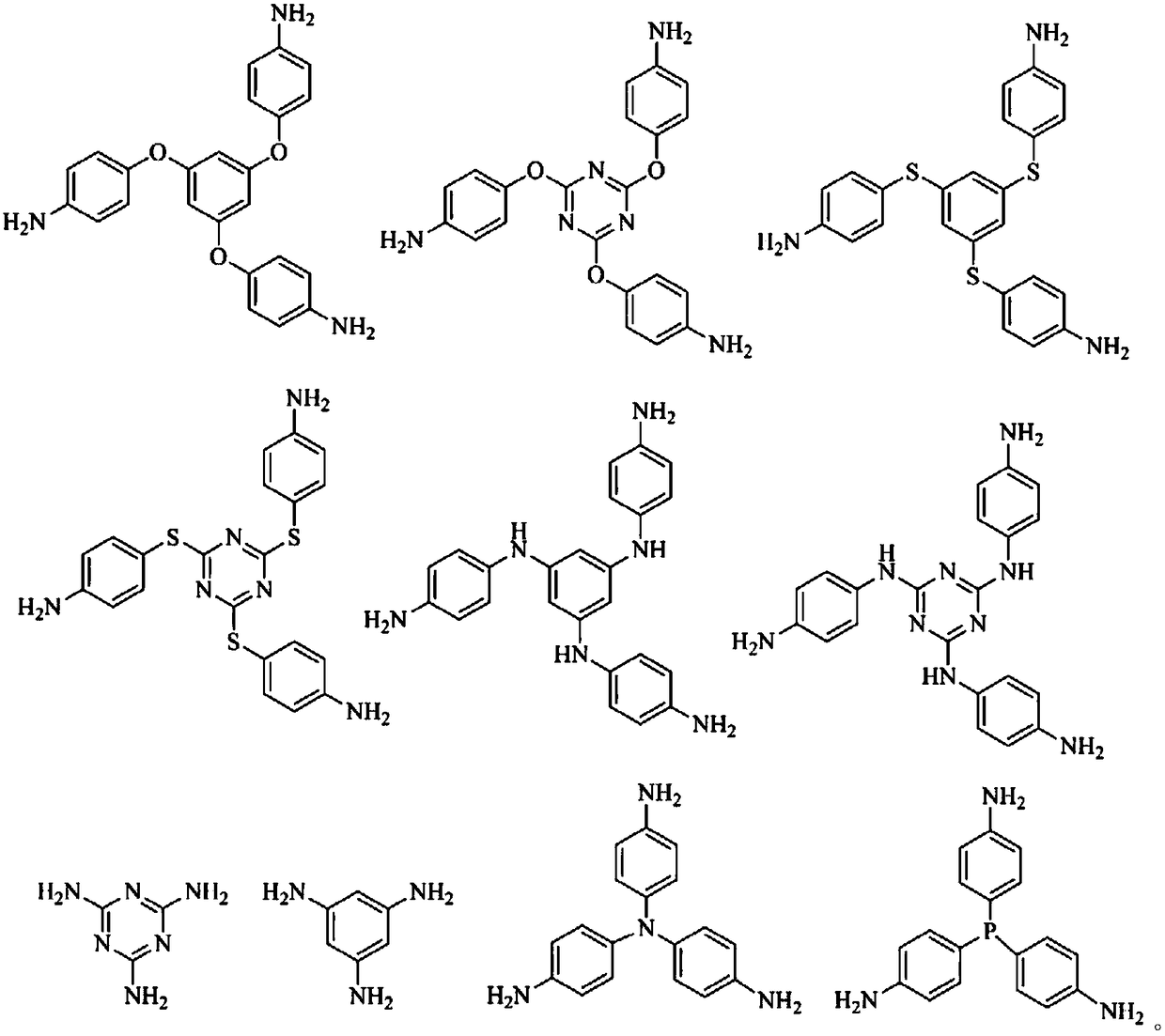
Image
Examples
Embodiment 1
[0026] Get 2mol 1,3,5-tri(4-aminophenoxy)benzene and 2mol pyromellitic dianhydride and place it in a four-necked flask equipped with a mechanical stirrer, a reflux condenser, a drying tube and an airway tube, and then add 20ml of tetrahydrofuran, stirred and mixed evenly, under nitrogen atmosphere, stirred and heated to 80°C for 2h reaction to obtain triphenoxyphenylenediaminophthalic acid monomer.
[0027] Add 30ml of N-methylpyrrolidone as a solvent and 2ml of pyridine as a catalyst to triphenoxyphenylenediaminophthalic acid monomer, raise the temperature to 120°C in the first stage, reflux for 2 hours, then raise the temperature to 180°C in the second stage for reflux reaction After 5h, the amino terminal hyperbranched resin was obtained. Then, after cooling the entire reaction system to room temperature, add 0.002 mol of free radical inhibitor hydroquinone and 0.2 mol of end-capping agent acryloyl chloride, and then continue to react at room temperature for 2 hours. After ...
Embodiment 2
[0033]Take 2mol melamine and 2mol cyclobutene tetra-acid dianhydride and place in a four-necked flask equipped with a mechanical stirrer, a reflux condenser, a drying tube and an airway tube, then add 10ml of m-cresol, stir and mix evenly, and place the mixture in a nitrogen atmosphere. , stirring and raising the temperature to 120° C. for 1 h to obtain a triazine-based diaminocyclobutenedioic acid monomer.
[0034] Add 1 mL of catalyst quinoline to the triazine-based diaminocyclobutene dioic acid monomer, heat up to 200° C. and reflux for 12 hours to obtain an amino-terminated hyperbranched resin. Then, after the whole reaction system was cooled to room temperature, 0.001 mol of free radical inhibitor p-methylhydroquinone and 0.1 mol of capping agent maleic anhydride were added, and then heated to 120° C. to continue the reaction for 1 h. After the reaction was completed, the The organic solvent was recovered by distillation under reduced pressure (vacuum degree 0.05 MPa) at ...
Embodiment 3
[0039] Take 2.5mol 2,4,6-tris(N-p-aminophenyl)-1,3,5-triazine and 2.5mol4,4'-(hexafluoroisopropylpropyl)-diphthalic anhydride In a four-necked flask equipped with a mechanical stirrer, a reflux condenser, a drying tube and an airway tube, 15ml of N-methylpyrrolidone was then added, and after stirring and mixing evenly, the reaction was stirred at room temperature for 6 hours under a nitrogen atmosphere to obtain triphenyl Triazinyl dihydrophthalic acid monomer.
[0040] Add 0.1 g of catalyst triphenyl phosphite and 0.2 g of calcium chloride to the triphenyltriazine-based dihydrophthalic acid monomer, raise the temperature to 120°C for 12 hours under reflux in the first stage, and raise the temperature in the second stage to Reflux reaction at 180°C for 8 hours, and in the third stage, the temperature was raised to 200°C for reflux reaction for 4 hours to obtain an amino-terminal hyperbranched resin. Then, after the whole reaction system was cooled to room temperature, 0.001 m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com