A leukocyte membrane-modified paclitaxel targeted sustained-release liposome and preparation method thereof
A leukocyte membrane and paclitaxel technology, applied in the direction of liposome delivery, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as allergic reactions, liposome damage, drug leakage, etc. Achieve the effects of reducing phagocytosis or ingestion, preventing excessive release, and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 1. A method for preparing leukocyte membrane-modified paclitaxel-targeted sustained-release liposomes, the method comprising the following steps:
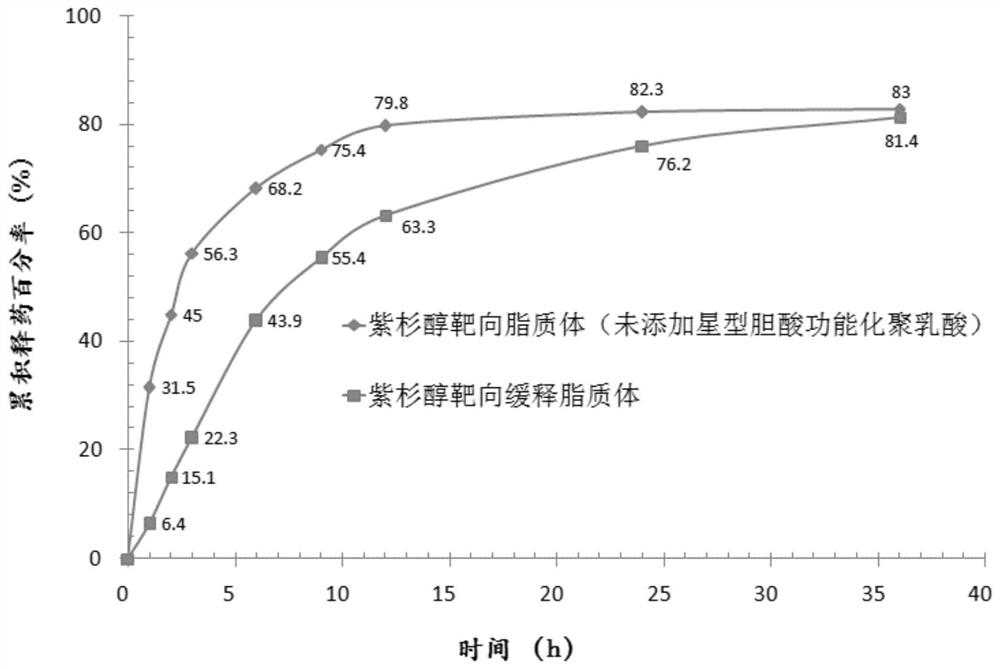
[0023] (1) Preparation of paclitaxel sustained-release liposomes: Weigh 5 mg of paclitaxel, 65 mg of soybean lecithin, 16 mg of cholesterol and 10 mg of star-shaped cholic acid-functionalized polylactic acid, dissolve them in a certain amount of chloroform, and evaporate under reduced pressure at 37°C Remove the solvent until a layer of liposome film is formed on the inner wall of the eggplant-shaped bottle, put it in a vacuum desiccator for 24 hours, add phosphate buffer (pH7.4), hydrate in a constant temperature water bath at 40°C for 1 hour, and ultrasonicate with an ice bath probe for 4-6 minutes , processed by a micro-jet high-pressure homogenizer (homogenization pressure is 120 MPa, homogenization temperature is 37° C., homogenization times is 5 times), and crosses a 0.22 μm microporous membrane to obtain paclitaxel sus...
Embodiment 2
[0053] 1. A method for preparing leukocyte membrane-modified paclitaxel-targeted sustained-release liposomes, the method comprising the following steps:
[0054] The preparation method is the same as in Example 1, wherein the dosage of each component is: 7.5 mg of paclitaxel, 60 mg of soybean lecithin, 20 mg of cholesterol, 7.5 mg of star-shaped cholic acid functionalized polylactic acid, and 5 mg of leukocyte membrane.
[0055] 2. Pharmacological characterization of paclitaxel-targeted sustained-release liposomes
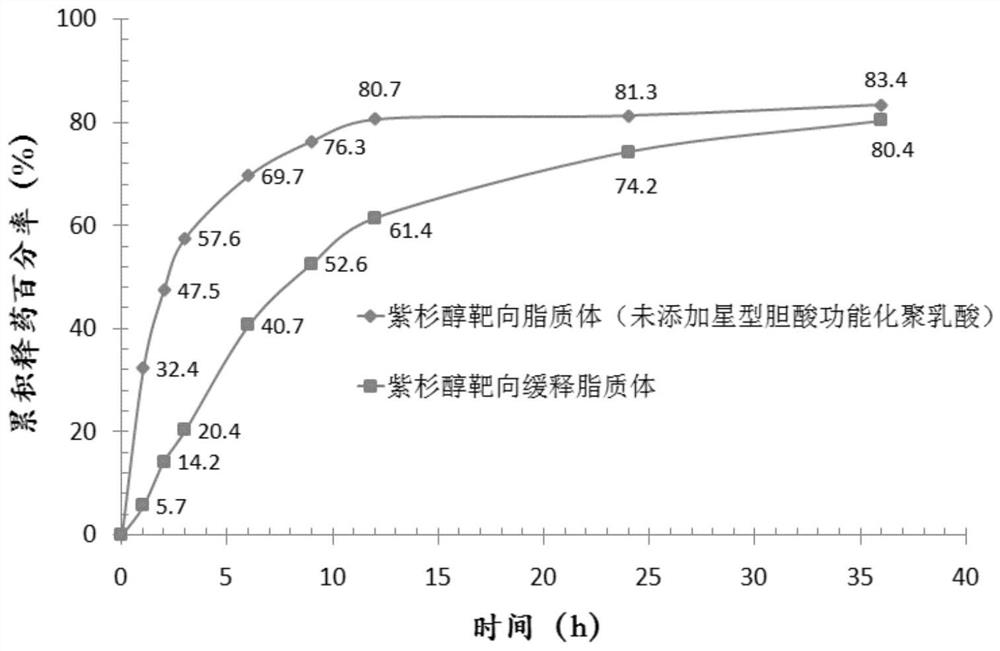
[0056] (1), particle size and polydispersity coefficient: adopt laser scattering particle size distribution analyzer to measure size, measurement result: average particle diameter is 87.5nm; Polydispersity coefficient is 0.186.
[0057] (2), surface topography: the measuring method is the same as that in Example 1, and the measurement result: the surface topography is spherical, and the size distribution is comparatively uniform.
[0058] (3), measurement and resu...
Embodiment 3
[0077] 1. A method for preparing leukocyte membrane-modified paclitaxel-targeted sustained-release liposomes, the method comprising the following steps:
[0078] The preparation method is the same as in Example 1, wherein the dosage of each component is: 10 mg of paclitaxel, 55 mg of soybean lecithin, 24 mg of cholesterol, 8 mg of star-shaped cholic acid functionalized polylactic acid, and 3 mg of white blood cell membrane.
[0079] 2. Pharmacological characterization of paclitaxel-targeted sustained-release liposomes
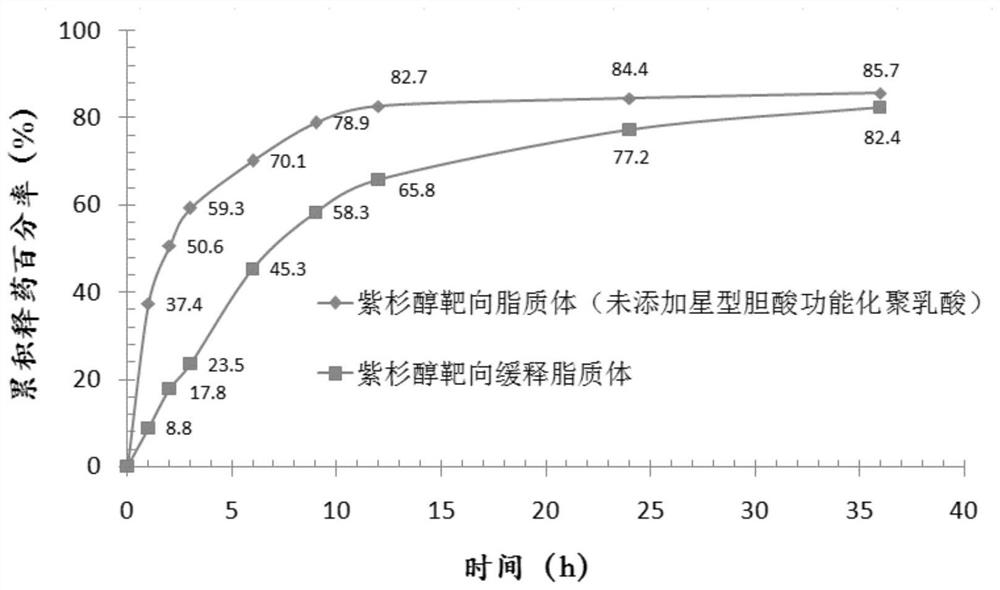
[0080] (1), particle size and polydispersity coefficient: adopt laser scattering particle size distribution analyzer to measure size. Measurement results: the average particle size is 84.9nm; the polydispersity coefficient is 0.177;
[0081] (2), surface topography: the measuring method is the same as that in Example 1, and the measurement result: the surface topography is spherical, and the size distribution is comparatively uniform.
[0082] (3), measuremen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com