Dimethyl benzyl alcohol hydrogenolysis catalyst and preparation method thereof
A technology of dimethyl benzyl alcohol and catalyst, which is applied in the field of catalysis, can solve the problems of blockage of catalyst carrier pores, decrease of catalyst use strength, and threats to the stable operation of industrial devices, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
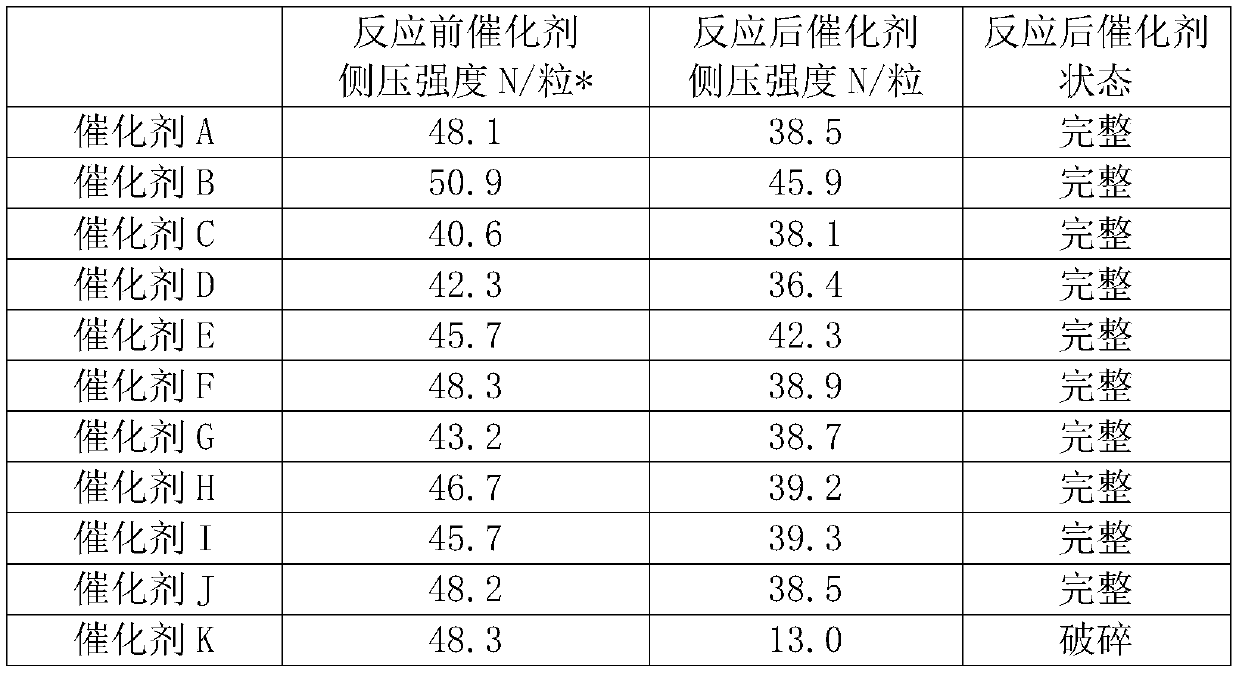
Image
Examples
Embodiment 1
[0081] (1) Add 300g water and 80.0g sodium silica sol (SiO in silica sol) into the reaction kettle 2 The content is 30wt%, SiO 2 The particle size is 30nm, the pH value is 9.0) and stirred evenly.
[0082] (2) 273.4g copper nitrate (Cu(NO 3 ) 2 ·3H 2 O, 242), 131.6g zinc nitrate (Zn(NO 3 ) 2 ·6H 2 O, 298), 63.6g magnesium nitrate (Mg(NO 3 ) 2 ·6H 2 (256), 3.0g lead nitrate (331) and 3.4g bismuth nitrate (395) are dissolved in 1226g water and prepare mixed salt solution, preparation concentration is the sodium carbonate aqueous solution of 15wt%, with above-mentioned two kinds of solutions heated to 60 ℃ respectively then Add it dropwise to the aforementioned reactor, the time of dropping is 40min, the temperature of the precipitation process is controlled at 60°C, and the pH value of the precipitation process is 7.0 (wherein, the consumption of the aqueous sodium carbonate solution is controlled according to the pH value of the precipitation process so that the metal ...
Embodiment 2
[0089] (1) Add 300g of water and 82.7g of sodium silica sol (SiO in silica sol) into the reaction kettle 2 The content is 30wt%, SiO 2 The particle size is 25nm, the pH value is 9.5) and stirred evenly.
[0090] (2) 303.7g copper nitrate, 102.4g zinc nitrate, 18.7g barium nitrate, 4.5g lead nitrate and 3.4g bismuth nitrate are dissolved in 1130g water to prepare mixed salt solution, preparation concentration is 15wt% sodium carbonate aqueous solution, above-mentioned two The solutions were heated to 45° C. and then added dropwise to the aforementioned reaction kettle in parallel. The dropping time was 30 min. The temperature of the precipitation process was controlled at 45° C., and the pH value of the precipitation process was 6.5 (wherein, the aqueous solution of sodium carbonate was controlled according to the pH value of the precipitation process. The amount used is 105% of the theoretically required amount to completely precipitate the metal particles), and then aged at ...
Embodiment 3
[0095] (1) Add 300g of water and 78g of sodium silica sol (SiO in silica sol) into the reaction kettle 2 The content is 30wt%, SiO 2 The particle size is 20nm, the pH value is 9.5) and stirred evenly.
[0096] (2) 334.1g copper nitrate, 102.4g zinc nitrate, 21.1g calcium nitrate (Ca(NO 3 ) 2 4H 2 (236), 3.0g lead nitrate and 5.1g bismuth nitrate are dissolved in 1225g water and prepare mixed salt solution, preparation concentration is the sodium carbonate aqueous solution of 15wt%, and above-mentioned two kinds of solutions are heated to 50 ℃ respectively and then co-current dropwise add to aforementioned In the reaction kettle, the dropping time is 50min, the temperature of the precipitation process is controlled at 50°C, and the pH value of the precipitation process is 6.8 (wherein, according to the pH value of the precipitation process, the amount of sodium carbonate aqueous solution is controlled to be 105% of the theoretically required amount of metal particles. %), a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com