Polysubstituted 1,4-diene compound and preparation method thereof
A technology of compound and diene, which is applied in the field of multi-substitution, can solve the problems of poor tolerance of coupling products to substrate groups, delay of synthesis efficiency, inability to achieve high functionalization of diene skeleton, etc., and achieve high yield High, easy to obtain raw materials, good economical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The synthesis of embodiment 11-phenylprop-2-en-1-alcohol
[0035] (1) Dissolve benzaldehyde in THF at -10°C, slowly add vinylmagnesium bromide Grignard reagent dropwise, stir at -10°C for 15 minutes, then continue stirring at room temperature for 4 to 8 hours to obtain a reaction mixture ; Wherein, the mol ratio of described benzaldehyde and vinylmagnesium bromide is 1:(1~2);
[0036] (2) Quenching the reaction with saturated ammonium chloride solution, extracting the reaction mixture, drying, and distilling off the solvent under reduced pressure, the residue was purified by silica gel chromatography with petroleum ether and ethyl acetate (PE / EA) to obtain a colorless Liquid 1-phenylprop-2-en-1-ol.
Embodiment 2
[0037] Synthesis of Example 21-phenyl-2-(triphenyl-λ5-phosphoryl)ethane-1-one
[0038] (1) Under reflux conditions, dissolve 2-bromoacetophenone and triphenylphosphine in THF, then continue to stir at reflux temperature for 6 to 12 hours to obtain a reaction mixture; wherein, the 2-bromoacetophenone The molar ratio with triphenylphosphine is 1:(1~1.5);
[0039] (2) Filter the above reaction mixture, wash the filter cake with ethyl acetate (EA), dissolve the filter cake with dichloromethane (DCM), add saturated sodium hydroxide solution, stir at room temperature for 30-60min, and use saturated Sodium chloride was separated into layers, the reaction mixture was extracted, dried, and the solvent was distilled off under reduced pressure to obtain 1-phenyl-2-(triphenyl-λ5-phosphoryl)ethan-1-one as a pale yellow solid.
Embodiment 3
[0041] (1) In a 10mL Shrek tube, under argon atmosphere, add 1-phenylprop-2-en-1-ol (0.36mmol), 2-(triphenyl-1,5-phosphorous) Ethyl acetate (0.3 mmol), barium bis(trifluoromethylsulfonyl)imide (5 mol%), [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride dichloromethane The complex (3mol%) was stirred and reacted at 100°C in dimethyl sulfoxide (2mL), and the reaction equation was:
[0042]
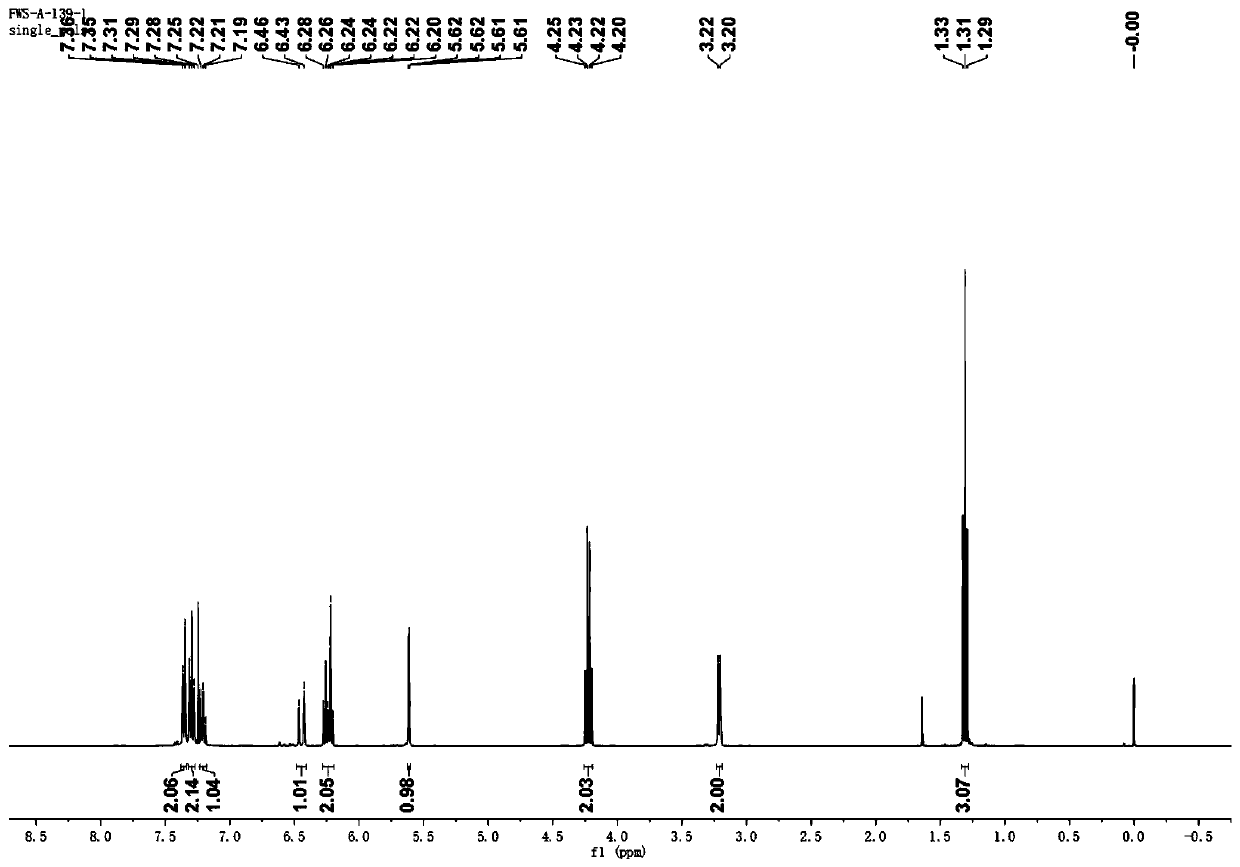
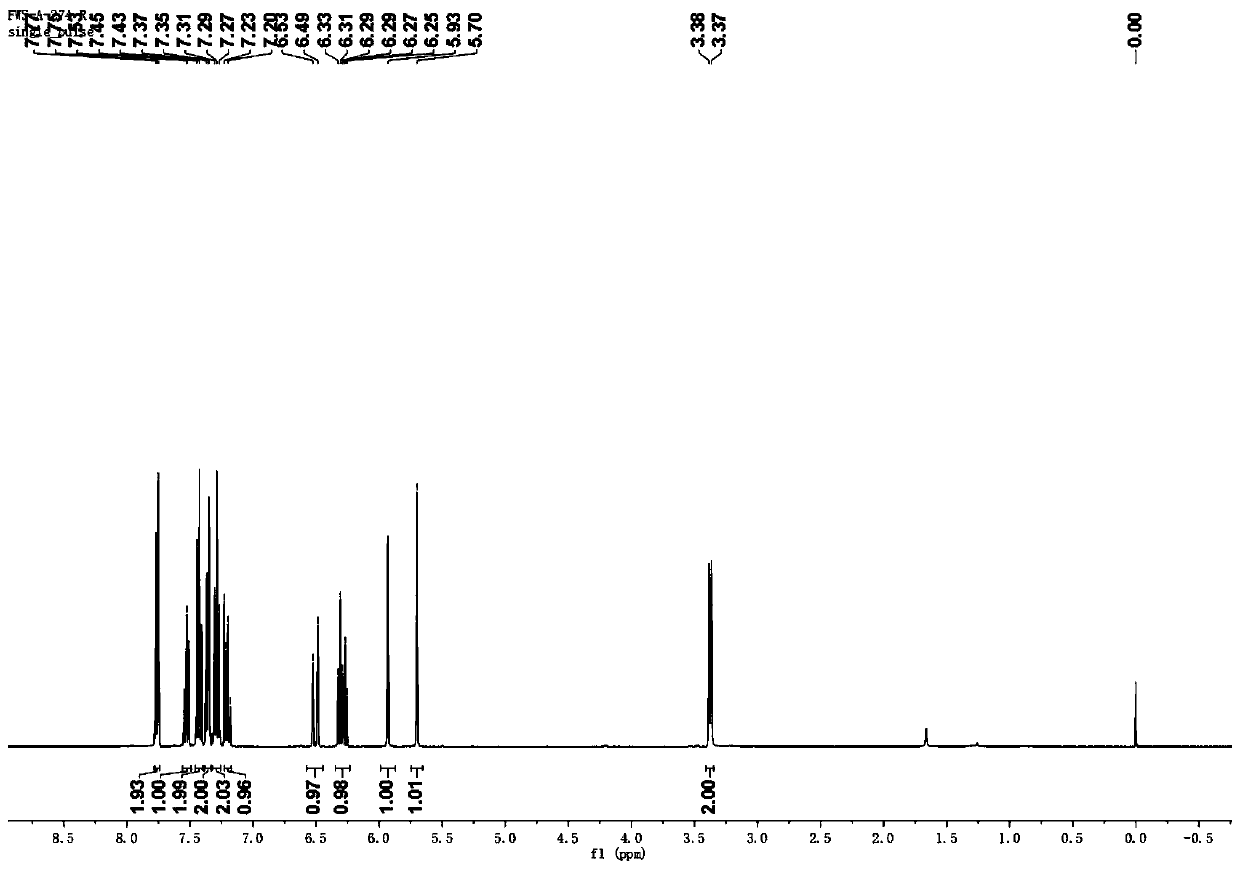
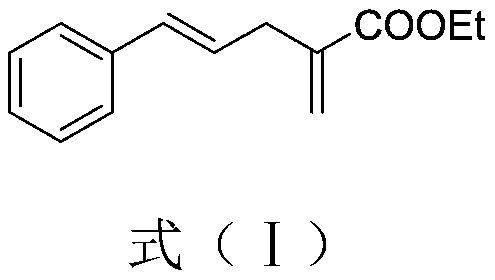
[0043] (2) After the reaction was carried out for 48 hours, an aqueous formaldehyde solution (37%, 0.5 mL) was added to the reaction mixture, and the reaction was continued at room temperature for 12 hours. After the reaction was completed, the reaction mixture was extracted with ethyl acetate and saturated brine. After drying, the ethyl acetate was distilled off under reduced pressure, and petroleum ether and ethyl acetate (PE / EA=9: 1) The residue was purified to obtain yellow liquid (E)-2-methylene-5-phenylpent-4-enoic acid ethyl ester with a yield of 90%. Wherein, the proton...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com