A kind of preparation method of flower-like metal fluoride nanomaterial
A nanomaterial and fluoride technology, applied in the field of nanomaterials, can solve the problems of large particle size, complex preparation method and high production cost of metal fluoride nanomaterials, and achieves high electrochemical activity and catalytic activity, cheap and easy-to-obtain raw materials, The effect of low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A method for preparing a flower-shaped ferric fluoride nanomaterial, comprising the following steps:
[0035] 1) Put the melamine in the crucible, slowly introduce the air flow, and raise the temperature to 550°C at a rate of 3°C / min to obtain a light yellow g-C 3 N 4 ;
[0036] 2) Add 100mg of g-C 3 N 4 Add 80mL of ethanol, ultrasonically disperse for 3h, get g-C 3 N 4 Dispersions;
[0037] 3) 660mg of FeCl 3 ·6H 2 O join g-C 3 N 4 In the dispersion, react at 70°C for 1h, then add 0.6mL of hydrofluoric acid with a concentration of 40wt%, and stir for 30min to obtain a mixed solution (FeCl 3 and the molar ratio of HF is 1:5);
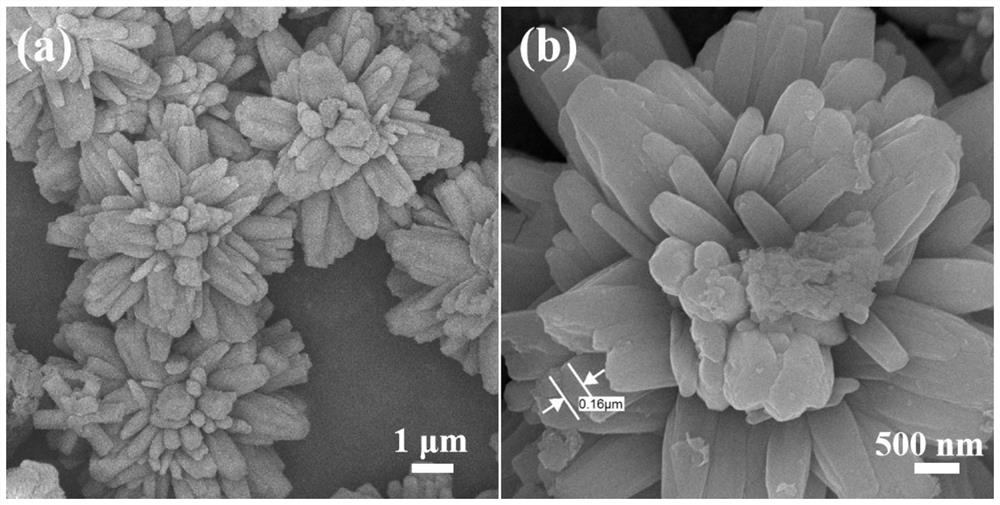
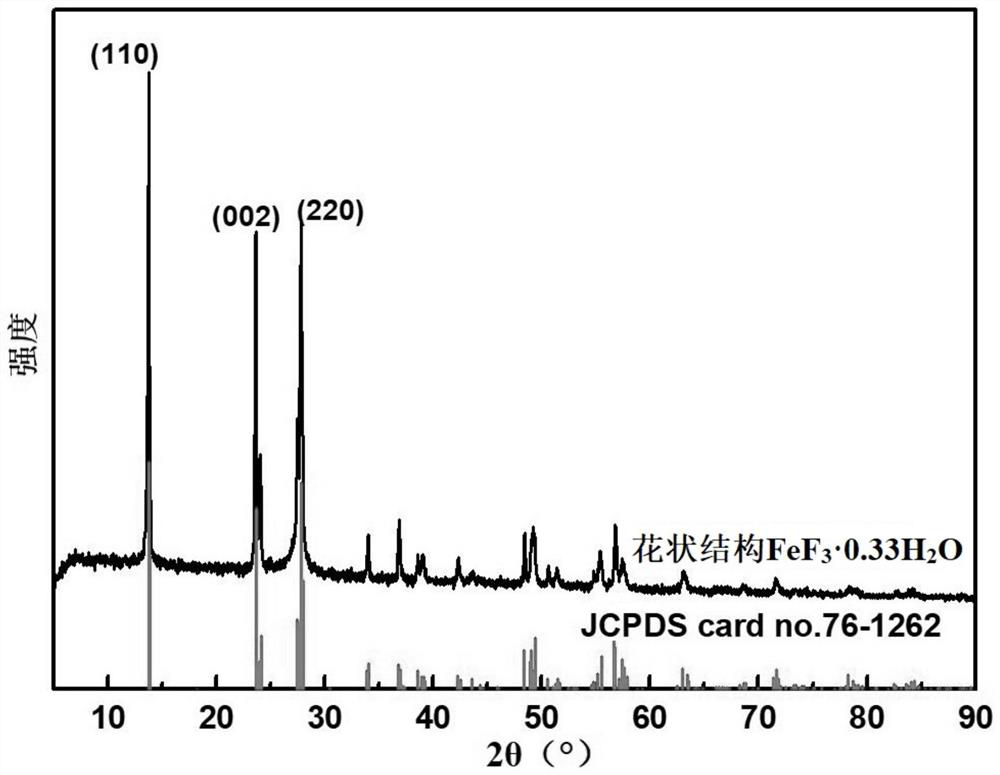
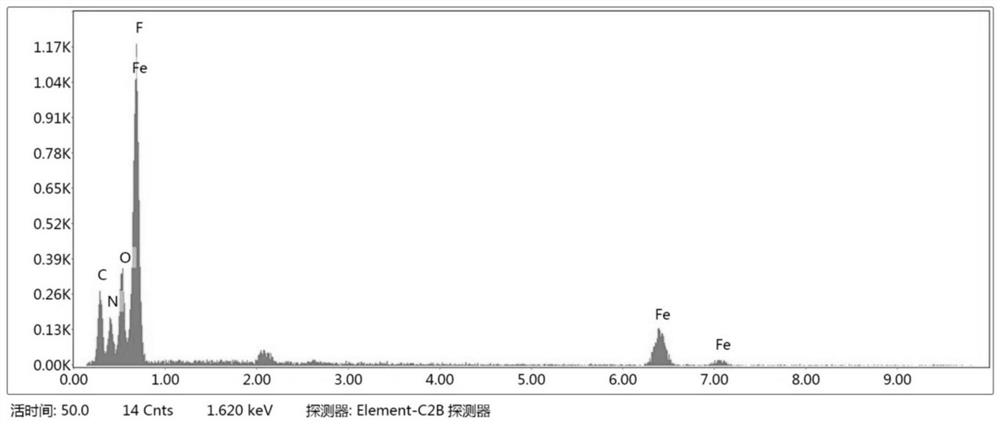
[0038] 4) Add the mixed solution into the reaction kettle, then seal the reaction kettle and place it in a blast oven, react at 180°C for 3 hours, take out the lining after the reaction kettle is cooled to room temperature, filter the reaction solution, and filter the filtered solid. Washed and dried to obtain the flower-like structur...
Embodiment 2
[0047] A method for preparing a flower-shaped ferric fluoride nanomaterial, comprising the following steps:
[0048] 1) Put the melamine in the crucible, slowly introduce the air flow, and raise the temperature to 550°C at a rate of 3°C / min to obtain a light yellow g-C 3 N 4 ;
[0049] 2) Add 100mg of g-C 3 N 4 Add 80mL of ethanol, ultrasonically disperse for 3h, get g-C 3 N 4 Dispersions;
[0050] 3) 660mg of FeCl 3 ·6H 2 O join g-C 3 N 4 In the dispersion, react at 70°C for 1h, then add 1.45mL of hydrofluoric acid with a concentration of 40wt%, and stir for 30min to obtain a mixed solution (FeCl 3 and the molar ratio of HF is 1:12);
[0051] 4) Add the mixed solution into the reaction kettle, then seal the reaction kettle and place it in a blast oven, react at 160°C for 6 hours, take out the lining after the reaction kettle is cooled to room temperature, filter the reaction solution, and filter the filtered solid Washed and dried to obtain the flower-like structu...
Embodiment 3
[0054] A method for preparing a flower-shaped ferric fluoride nanomaterial, comprising the following steps:
[0055] 1) Put the melamine in the crucible, slowly introduce the air flow, and raise the temperature to 550°C at a rate of 3°C / min to obtain a light yellow g-C 3 N 4 ;
[0056] 2) Add 100mg of g-C 3 N 4 Add 80mL of ethanol, ultrasonically disperse for 3h, get g-C 3 N 4 Dispersions;
[0057] 3) 660mg of FeCl 3 ·6H 2 O join g-C 3 N 4 In the dispersion, react at 70°C for 1 h, then add 0.6 mL of hydrofluoric acid with a concentration of 40 wt%, and stir for 30 min to obtain a mixed solution;
[0058] 4) Add the mixed solution into the reaction kettle, then seal the reaction kettle and place it in a blast oven, react at 200°C for 2 hours, take out the lining after the reaction kettle is cooled to room temperature, filter the reaction solution, and filter the filtered solid Washed and dried to obtain the flower-like structure FeF 3 0.33H 2 O.
[0059] After tes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com