Subunit nano vaccine for preventing and treating nasopharyngeal carcinoma and preparation method of subunit nano vaccine
A nanoparticle and immune adjuvant technology, applied in the field of biomedicine, can solve the problems of ineffective immune effect and weak immunogenicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1 Preparation of EBNA1△GA protein
[0082] Refer to the amino acid sequence of wild-type EBNA1 nuclear antigen protein of 4 strains of human gamma herpesvirus B95-8 (NCBI database accession number: YP_401677.1);
[0083] (1) Synthesize the target gene fragment truncated from the 92-327 sequence of the full-length EBNA1, and the C-segment is fused with a 6x histidine tag and a stop codon.
[0084](2) The synthetic gene fragment was subcloned into the prokaryotic expression vector pET30a using Ndel and Hind III as sites, and transformed into BL21 (DE3) strain.
[0085] (3) In the TB medium containing kanamycin, culture on a shaker at 37°C, when the OD600 reaches about 1.2, add IPTG, and continue to stimulate culture at 37°C for 4 hours.
[0086] (4) Collect bacteria by centrifugation, add dissolution buffer and ultrasonic-assisted bacteriostasis, centrifuge to save the supernatant, add guanidine hydrochloride to denature the target protein.
[0087] (5) The targ...
Embodiment 2
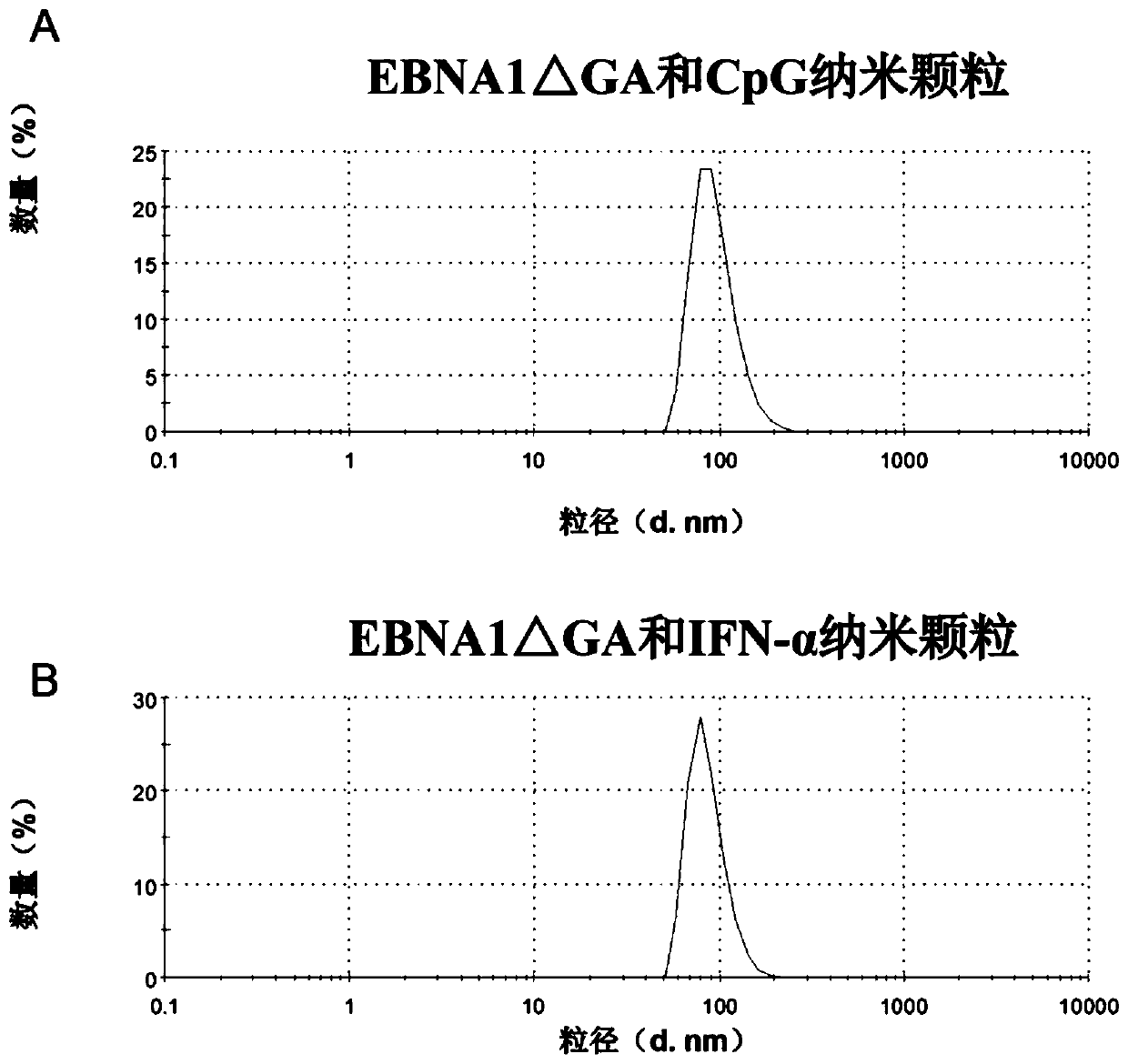
[0088] The preparation of embodiment 2 nanoparticles
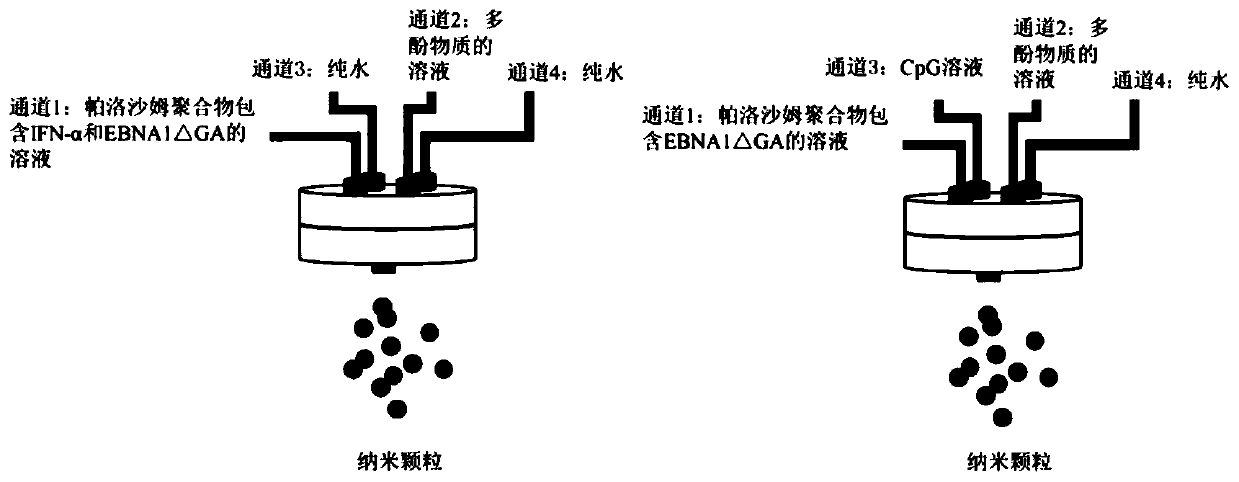
[0089] 1. Preparation of nanoparticles
[0090] 1. Reagent: EBNA1△GA protein is expressed by E. coli expression system, and then obtained through dissolution and renaturation. Its amino acid sequence is shown in SEQ ID NO:1.
[0091] CpG ODN1826 is commercially available, and its nucleotide sequence is shown in SEQ ID NO:2.
[0092] All other reagents were purchased commercially.
[0093] 2. Preparation process:
[0094] (1) PF-127 (average molecular weight 12.6KDa) was dissolved in ultrapure water, and dissolved under magnetic stirring to obtain 0.3 mg / mL of PF-127.
[0095] (2) Disperse tannic acid (TA) in ultrapure water, and obtain a TA concentration of 0.2 mg / mL under magnetic stirring.
[0096] (3) EBNA1△GA protein was dissolved in ultrapure water, and the pH was adjusted to 4.1 with 1M hydrochloric acid to obtain a concentration of EBNA1△GA protein of 71.43 μg / mL.
[0097] (4) CpG ODN1826 was dissolved in ultra...
Embodiment 3
[0126] Example 3 Construction of murineized nasopharyngeal carcinoma cells stably expressing EBNA1 antigen
[0127] 1. Reagents: The vector plasmid pBABE-Puro, the packaging plasmid pVSVG, and phit60 are original in the laboratory. The transfection reagent lipo2000 and puromycin were purchased commercially.
[0128] 2. Construction process:
[0129] (1) The vector plasmid pBABE-Puro-EBNA1 containing the full-length sequence of EBNA1 and the vector plasmid pBABE-Puro-EBNA1-EGFP fused with green fluorescent protein were constructed using EcoRI and BamHI sites.
[0130] (2) Using the pBABE-Puro-EBNA1-EGFP plasmid, 293T cells were transiently transfected with lipo2000, and the expression of EGFP was observed by fluorescence microscopy after 24 hours.
[0131] (3) Use the vector plasmid pBABE-Puro-EBNA1 constructed in step 1, together with the packaging plasmid pVSVG, phit60. The mass ratio of pBABE-Puro-EBNA1 or pBABE-Puro-EBNA1-EGFP:pVSVG:phit60 is 1:1:1, 2:2:3 or 3:2:2. With t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com