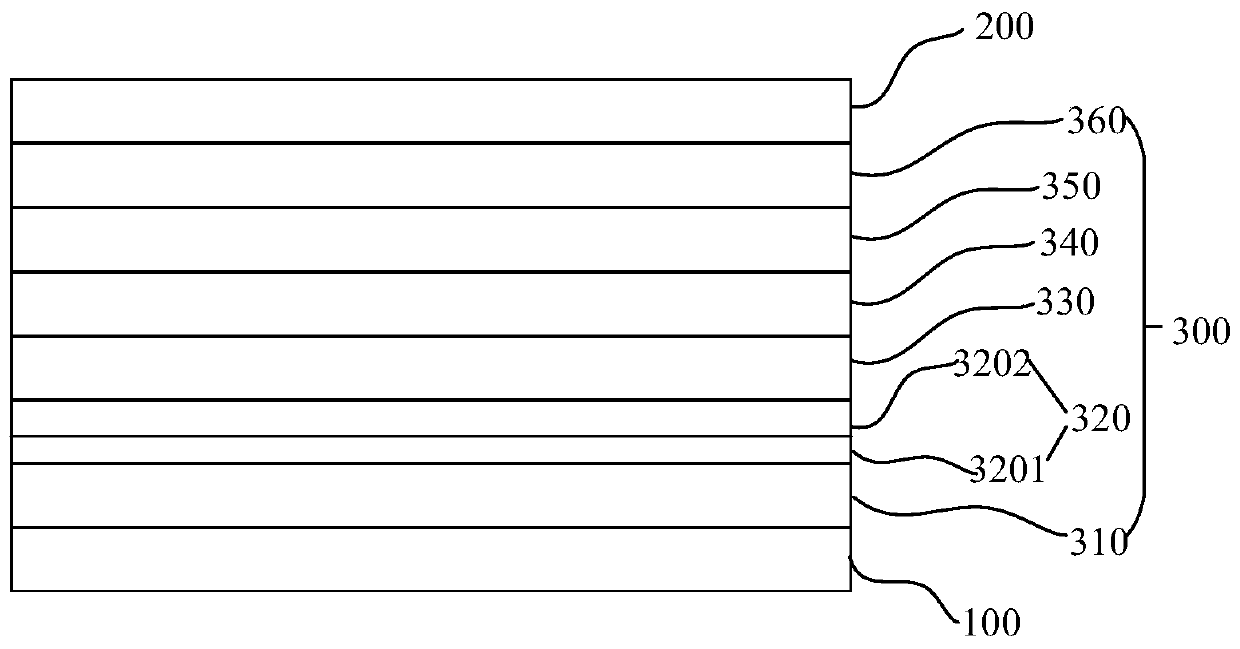
Organic compound, electronic element comprising same, and electronic device
A technology of organic compounds and chemical formulas, applied in the field of organic compounds, which can solve the problems of rising driving voltage, lowering luminous efficiency, shortening lifespan, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0227] In this specification, all temperatures are in degrees Celsius unless otherwise stated.
[0228] In this specification, unless otherwise stated, all reagents used in the examples were purchased from commercial suppliers and used without further purification.
[0229] Anhydrous tetrahydrofuran, dioxane, and toluene ethyl ether used in the examples of this specification were all obtained by reflux drying of metal sodium; anhydrous dichloromethane and chloroform were obtained by reflux drying of calcium hydride; ethyl acetate, petroleum ether, n-hexane Alkanes, N,N-dimethylacetamide and N,N-dimethylformamide were dried over anhydrous sodium sulfate before use.
[0230] In the examples of this specification, unless otherwise stated, the reaction is generally carried out under nitrogen or argon positive pressure or on an anhydrous solvent with a drying tube, a suitable rubber stopper on the reaction bottle, and the substrate is injected into the reaction via a syringe. , gl...
preparation example 1
[0231] Preparation Example 1: Synthesis of Compound 15
[0232] 1) Synthesis of Intermediate-1
[0233] Add 100g (that is, 373.5mmol) of 2-chloro-4,6-diphenyl-1,3,5-triazine into a three-necked flask filled with 1L tetrahydrofuran (THF), and add 25.12g of it dropwise at -78°C (i.e. 392.20mmol) n-butyllithium (n-Buli), after the dropwise addition, keep warm for 1h, then add 40.85g (i.e. 1120.5mmol) trimethyl borate dropwise, continue to keep warm for 1h, then warm up to room temperature and stir overnight. Add hydrochloric acid (2 mol / L) to adjust the pH to neutral, obtain a white crude product by filtration, and slurry with n-heptane to obtain 72.1 g of white solid Intermediate-1 (yield 70%).
[0234]
[0235] 2) Synthesis of Intermediate 2
[0236]
[0237] 2.1) Synthesis of intermediate 2-1
[0238] 100g (ie 315.1mmol) of 2-bromo-4-chloro-1-iodobenzene, 49.27g (ie 315.1mmol) p-chlorophenylboronic acid, 18.21g (ie 15.7mmol) tetrakis (triphenylphosphine) palladium (Pd...
preparation example 2
[0250] Preparation Example 2: Synthesis of Compound 17
[0251] Compound 17 was synthesized using the same method as in Preparation 1 (yield 76%, LC-MS (ESI, pos.ion): m / z=749.3 ([M+H] + )), the difference is that the following raw materials 1 and raw materials 2 are used to replace 2-bromo-4-chloro-1-iodobenzene and p-chlorophenylboronic acid used to prepare intermediate 2 in Preparation 1, thus obtaining the following intermediate Body 3-1, Intermediate 3-2 and Intermediate 3.
[0252]
[0253]
[0254] Compound 17 prepared by the above method is shown below:
[0255]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com