Detection method of macrolide antibiotics in honey and its sample processing method
A macrolide and sample processing technology, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of large detection errors, complex components, sample detection, etc., and achieve high automation, good detection effect and high recovery rate high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Take 400mgGO and ultrasonically disperse in 100mL water for 30min to make the GO disperse evenly and obtain the GO dispersion liquid;
[0047] 1. Dissolve 10g of zinc acetate dihydrate in 10mL of water to obtain liquid A, pour liquid A into the GO dispersion, stir for 1 hour and mix well to obtain a mixed liquid;
[0048] 3. Dissolve 28g of 2-methylimidazole in 10mL of water to obtain liquid B, pour liquid B into the above mixture, continue to stir and react at 20°C overnight (16h), centrifuge, discard the supernatant, and wash the residue with methanol several times Wash to remove unreacted substances, and dry the remaining solid at 60°C for 8 hours to obtain an off-white solid, which is fully ground to obtain ZIF-8@GO powder, which is designated as ZIF-8@GO-1.
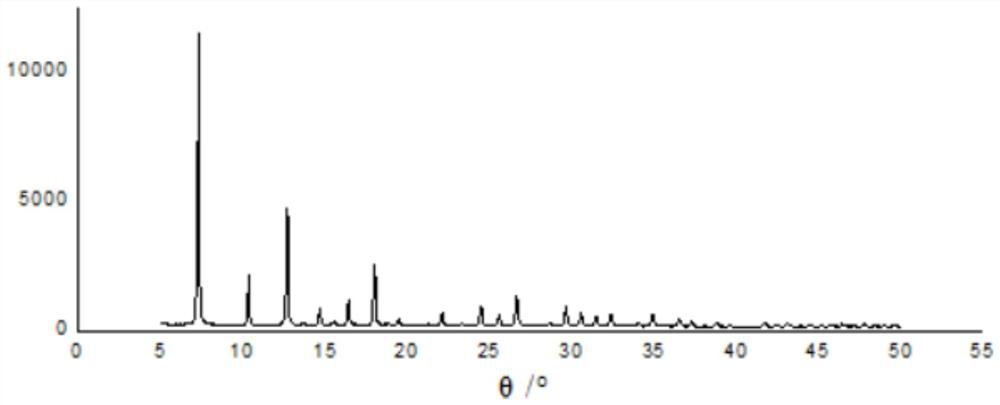
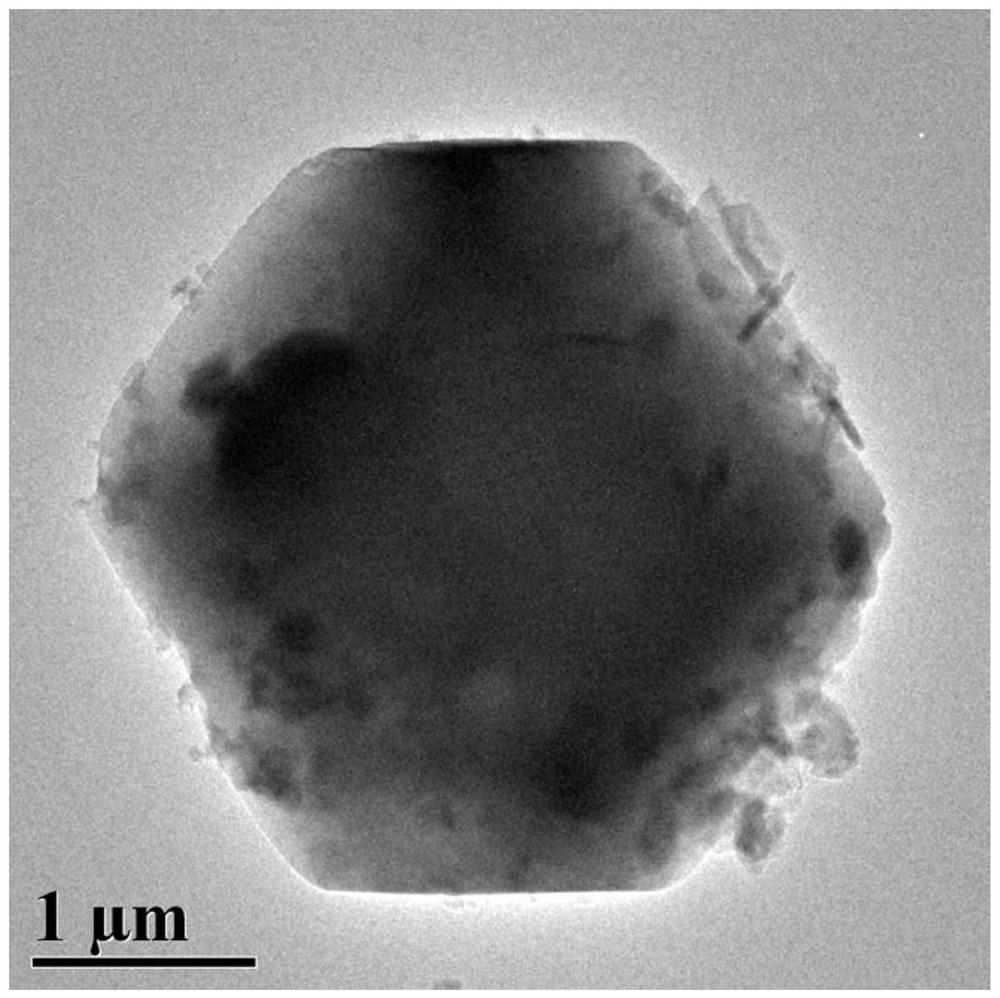
[0049] The particle size of ZIF-8@GO-1 powder is 1μm-1.1μm, and the particle size is relatively large. The XRD characteristic diagram of ZIF-8@GO-1 is shown in figure 1 , which proved that ZIF-8 was tightly ...
Embodiment 2
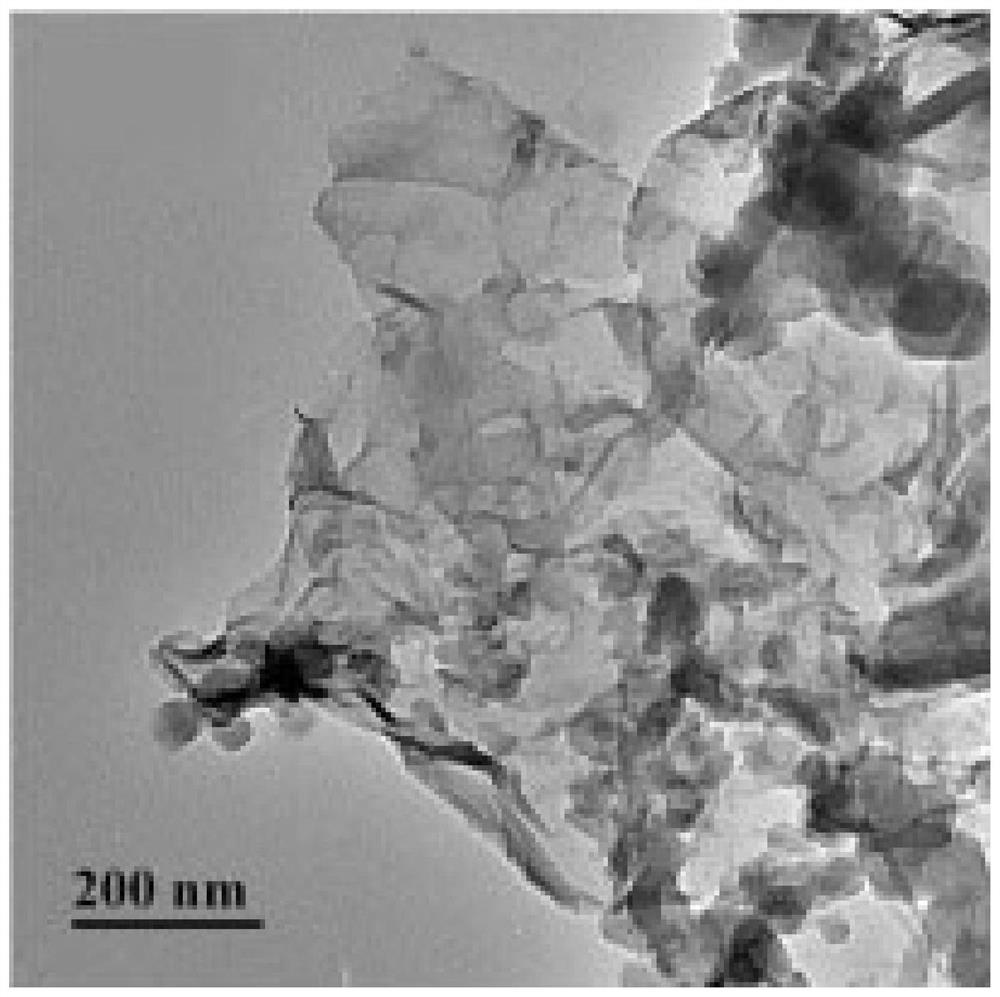
[0051] Except that the amount of GO was 500mg, the rest of the operation was the same as in Example 1 to obtain ZIF-8@GO powder, which was designated as ZIF-8@GO-2. The particle size of ZIF-8@GO-2 powder is 1μm-1.1μm, and the particle size is relatively large. XRD characteristic map of ZIF-8@GO-2 and figure 1 Similarly, it was proved that ZIF-8 was tightly combined with GO, and ZIF-8@GO was successfully synthesized. The TEM image of ZIF-8@GO-2 shows that GO is embedded in the pores of ZIF-8 crystals and / or attached to the surface of ZIF-8 crystals, but not ZIF-8 crystals are attached to the surface of GO.
Embodiment 3
[0053] Take 400mgGO and ultrasonically disperse in 100mL water for 30min to make the GO disperse evenly and obtain the GO dispersion liquid;
[0054] 1. Dissolve 10g of zinc acetate dihydrate in 10mL of water to obtain liquid A, pour liquid A into the GO dispersion, stir for 1 hour and mix well to obtain a mixed liquid;
[0055] Dissolve 3.5g of 2-methylimidazole in 10mL of water to obtain liquid B, pour liquid B into the above mixture, continue to stir and react overnight (20h) at 30°C, centrifuge, discard the supernatant, and wash the residue with methanol several times Wash to remove unreacted substances, and dry the remaining solid at 55°C for 12 hours to obtain an off-white solid, which is fully ground to obtain ZIF-8@GO powder, which is designated as ZIF-8@GO-3.
[0056] The particle size of ZIF-8@GO-3 powder is 1μm-1.1μm, and the particle size is relatively large. The XRD characteristic map of ZIF-8@GO-3 and figure 1 Similarly, it was proved that ZIF-8 was tightly com...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com