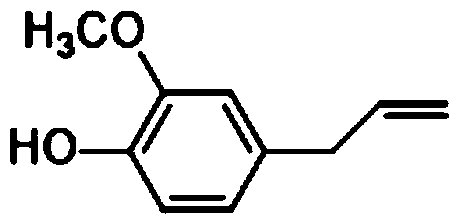
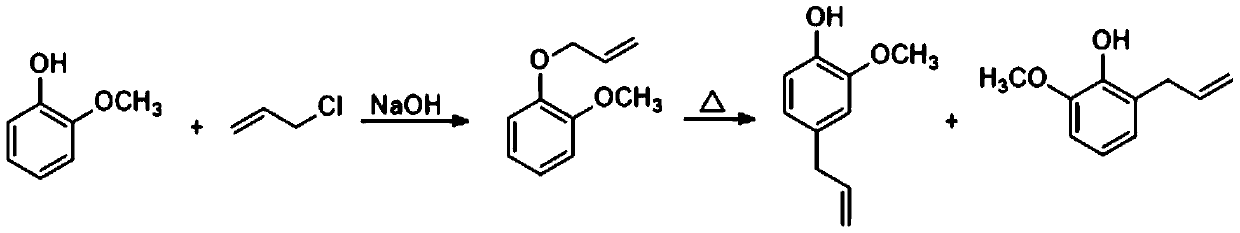
Synthetic method of eugenol
A synthesis method and eugenol technology are applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., and can solve problems such as increased production cost, pollution, and difficulty in wastewater treatment, and achieve increased yield, reduced production cost, and reaction mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
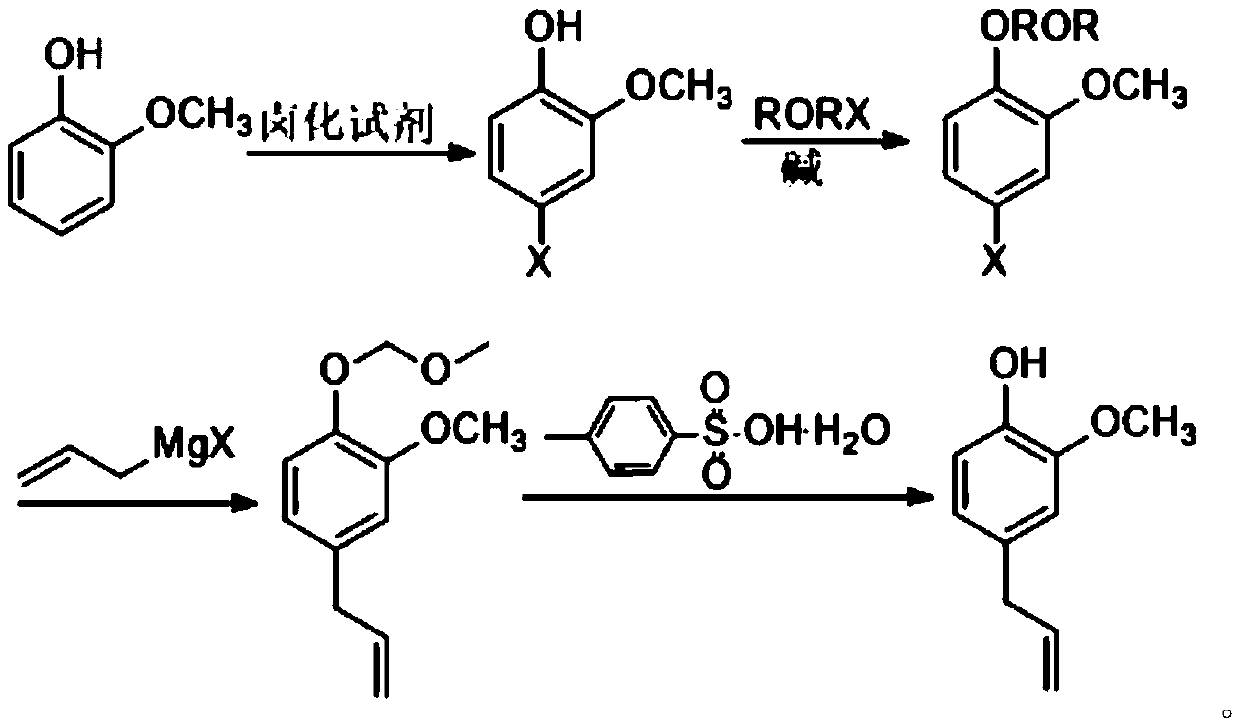
[0035] 1. Add 12.40 grams (0.10mol) of guaiacol, 20.25 grams (0.15mol) of sulfuryl chloride and 60ml of toluene into a 200ml round bottom flask, stir and react at 20°C, and absorb the tail gas with sodium hydroxide solution; after 6 hours of reaction Stop heating, add saturated sodium bicarbonate solution to the reaction mixture to neutralize to pH = 6-7, separate the toluene layer, dry over anhydrous sodium sulfate, filter, and distill toluene under reduced pressure to obtain 4-chloro-2-methoxy 14.27 g (0.09 mol) of base phenol, yield 90%.
[0036] 2. Add 15.86 grams (0.10mol) of 4-chloro-2-methoxyphenol, 15.18 grams (0.15mol) of triethylamine and 80ml methylene chloride in a 200ml round bottom flask, and add 12.08 grams of methyl chloromethyl ether dropwise. gram (0.15 mol). After the dropwise addition was completed, the reaction was stirred at room temperature for 12 hours, then 100ml of water was added to the reaction mixture, the dichloromethane layer was separated, the ...
Embodiment 2
[0041] 1. Add 12.40 g (0.10 mol) of guaiacol, 0.16 mol of thionyl chloride and 70 ml of dichloromethane into a 200 ml round bottom flask, stir and react at 25°C, and absorb the tail gas with sodium hydroxide solution. Stop heating after reacting for 7 hours, add saturated sodium bicarbonate solution to the reaction mixture to neutralize to pH = 6-7, separate the dichloromethane layer, dry over anhydrous sodium sulfate, filter, and distill off the dichloromethane under reduced pressure to obtain 14.43 g (0.091 mol) of 4-chloro-2-methoxyphenol, yield 91%.
[0042] 2. Add 15.86 g (0.10 mol) of 4-chloro-2-methoxyphenol, 0.16 mol of ethyl diisopropylamine and 80 ml of dichloroethane in a 200 ml round bottom flask, add ethyl-2-chloro Ethyl ether 0.16mol. After the dropwise addition was completed, the reaction was stirred at room temperature for 13 hours, then 120ml of water was added to the reaction mixture, the dichloroethane layer was separated, the water layer was extracted once...
Embodiment 3
[0047] 1. Add 12.40 g (0.10 mol) of guaiacol, 0.18 mol of thionyl chloride and 80 ml of dichloroethane into a 200 ml round bottom flask, stir and react at 22 ° C, and absorb the tail gas with sodium hydroxide solution. Stop heating after reacting for 6.5 hours, add saturated sodium bicarbonate solution to the reaction mixture to neutralize to pH = 6-7, separate the dichloroethane layer, dry over anhydrous sodium sulfate, filter, and distill off dichloroethane under reduced pressure Afterwards, 14.59 g of 4-chloro-2-methoxyphenol was obtained with a yield of 92%.
[0048] 2. Add 15.86 g (0.10 mol) of 4-chloro-2-methoxyphenol, 0.14 mol of potassium carbonate and 75 ml of toluene into a 200 ml round bottom flask, and add 0.14 mol of methyl bromomethyl ether dropwise. After the dropwise addition was completed, the reaction was stirred at room temperature for 11.5 hours, then 95ml of water was added to the reaction mixture, the toluene layer was separated, the water layer was extra...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com