Escherichia coli for expressing tyrosine phenol lyase active inclusion body and application of escherichia coli
A technology of tyrosine phenol and recombinant Escherichia coli, which is applied in the field of bioengineering, can solve problems such as environmental pollution, complicated process, and high cost, and achieve good application prospects, simple construction method, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
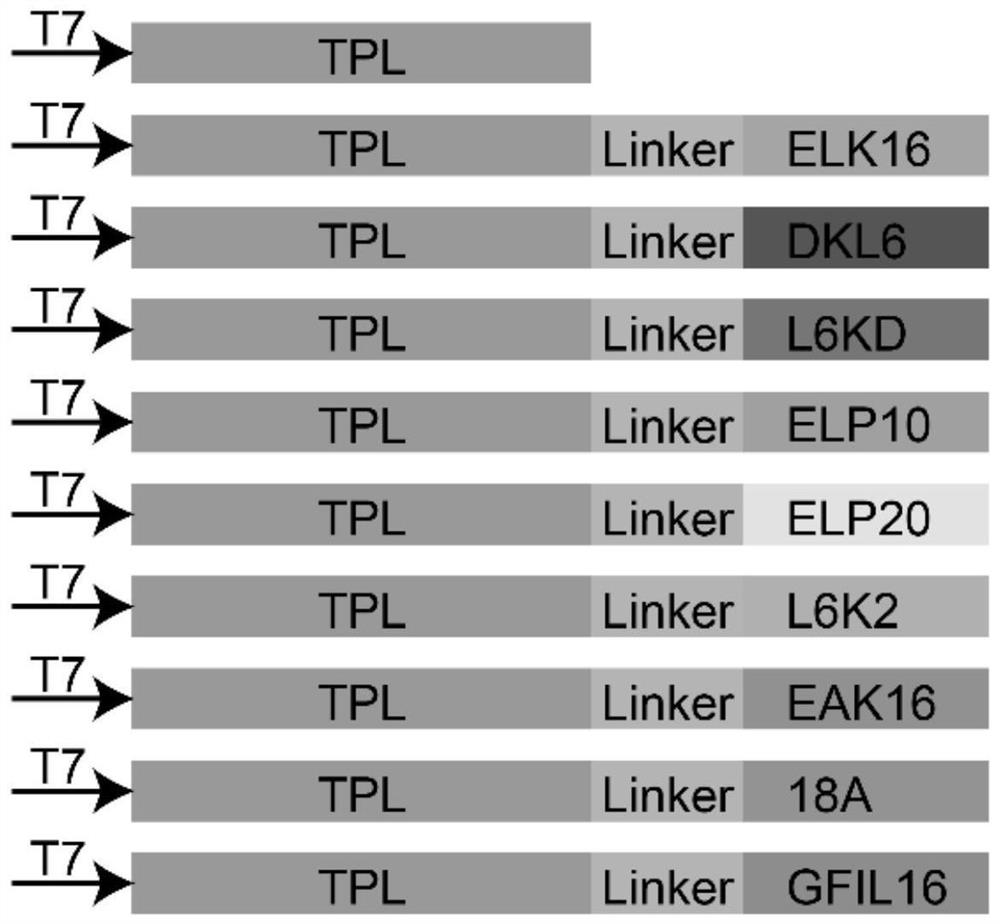
[0057] Example 1 Construction of recombinant plasmid pET-28(a)-TPL-peptide fused with functional short peptide.
[0058] Using the primer pET-28-TPL-F / R shown in Table 2, the plasmid pET-28 (a)-TPL (construction method reference publication number is CN109897845A patent application) is carried out linearization by polymerase chain reaction (PCR). PCR amplification, PCR conditions: 95°C pre-denaturation for 3min; 98°C denaturation for 30s; 55°C annealing for 15s; 72°C extension for 6min, 30 cycles of reaction; final extension at 72°C for 5min, PCR products were subjected to 1% agarose gel electrophoresis Detection and recovery by DNA purification kit. Nine short peptides (shown in Table 1) ELK16, DLK6, L6KD, Linker-ELP10, ELP20, L6K2, EAK16, 18A and GFIL16 were synthesized by Wuxi Tianlin Biotechnology Co. Primers TPL-ELK16-F / R, TPL-DLK6-F / R, TPL-L6KD-F / R, TPL-ELP10-F / R, TPL-ELP20-F / R, TPL-L6K2-F / R, TPL -EAK16-F / R, TPL-18A-F / R and TPL-GFIL16-F / R amplify the synthesized short ...
Embodiment 2
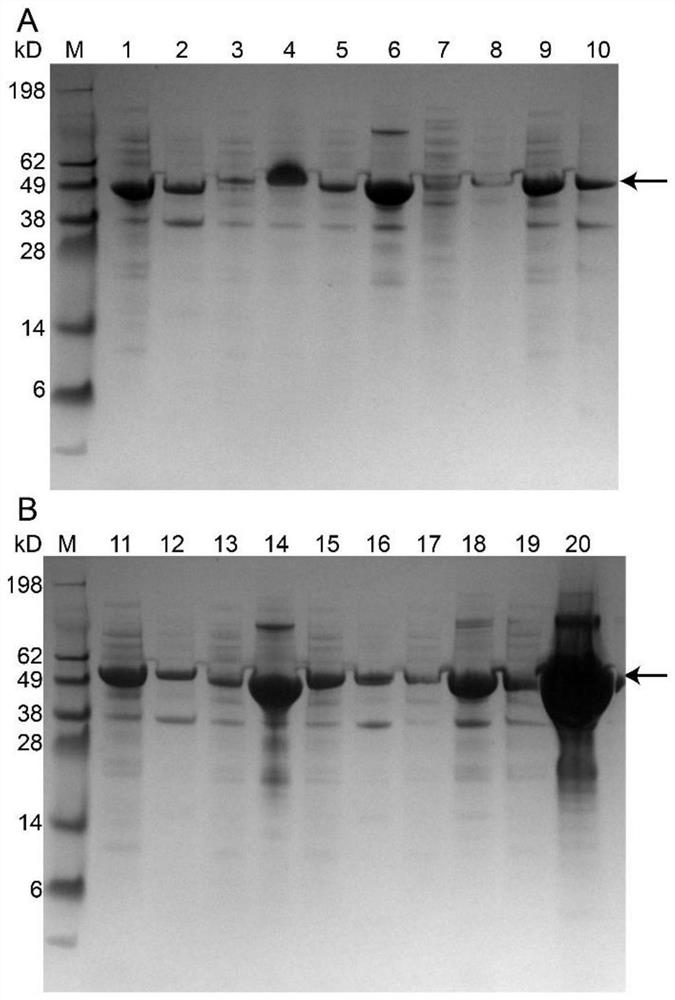
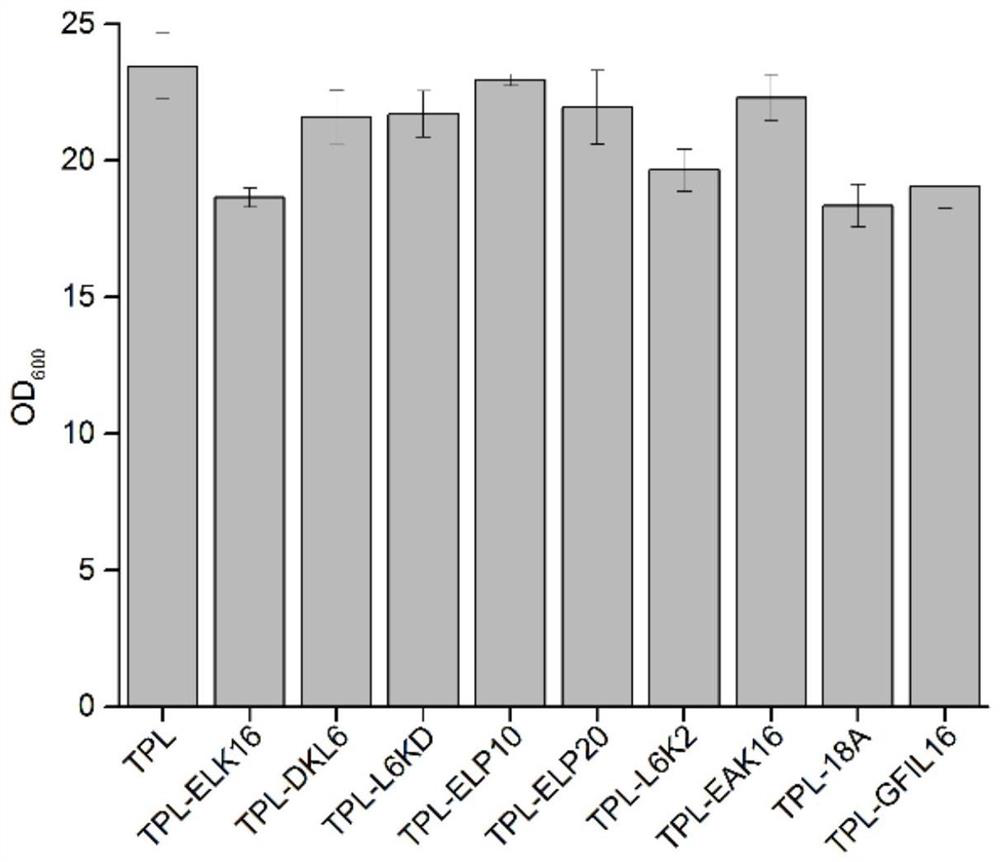
[0060] The expression situation of the tyrosine phenol lyase of embodiment 2 recombinant escherichia coli
[0061] The recombinant Escherichia coli TPL-ELK16, TPL-DLK6, TPL-L6KD, TPL-ELP10, TPL-ELP20, TPL-L6K2, TPL-EAK16, TPL-18A and TPL-GFIL16 constructed in Example 1 were respectively placed in TB medium After culturing for 3 hours, 0.2 mM IPTG as an inducer was added, and the temperature was lowered to 20° C., and the fermentation was continued for 10 hours. The fermentation broth was centrifuged at 8000rpm for 3min to collect the thalli, and PB buffer (pH 8.5, 50mM KH 2 PO 4 -K 2 HPO 4 ) Wash the cells 2-3 times, sonicate until the bacterial liquid is completely broken and become transparent, centrifuge at 9000rpm for 3min, and collect the supernatant and precipitate respectively. The intracellular supernatant fragment was purified by nickel column Ni-NTA Superflow Cabridge (5mL) and AKTA purifier, and the purified protein solution was collected and desalted and purifi...
Embodiment 3
[0063] TPL chemical properties and thermostability expressed by different strains of embodiment 3
[0064] The catalytic activity of the enzyme expressed by the recombinant bacteria constructed in Example 2 was measured under different pH environments, and the TPL enzyme activity was measured by the racemization reaction of tyrosine, and an enzyme activity unit was defined as decomposing and producing 1 μmol of sodium pyruvate per minute The amount of reaction substrate solution prepared by using 50mM potassium dihydrogen phosphate-dipotassium hydrogen phosphate (pH 6-8) and 50mM glycine-sodium hydroxide (pH 8-10) buffer solution, 100 μmol of tyrosine solution, to 900 μL Add 100 μL of pure enzyme solution (containing 30 μmol cofactor PLP) to the reaction substrate to activate the enzyme to catalyze the reaction. The reaction pH range is 6-10, the reaction temperature is 20°C, and it is protected from light. After 10 minutes of reaction, add 100 μL of 2M to the reaction solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com