Method for determining content of trimethoprim and various sulfonamides in medicine and application of method
A technology of trimethoprim and medicines, applied in the field of quality analysis of medicines, can solve the problems of undisclosed content detection methods, unfavorable qualitative and quantitative analysis, cumbersome operation steps, etc., and achieves simple and fast preparation operations, short measurement time, and reduced pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] The concrete method of content determination of compound sulfadiazine sodium injection comprises the following steps:
[0092] (1) Prepare mobile phase and set chromatographic conditions
[0093] The mobile phase is 0.1% phosphoric acid solution-acetonitrile, and the gradient elution program is as follows:
[0094]
[0095]
[0096] Set the chromatographic conditions as measuring wavelength 240nm, flow rate as 1.0ml / min, injection volume as 10μl, chromatographic column as C 18 (octadecylsilane bonded silica gel column);
[0097] (2) Preparation of standard stock solution and standard working solution
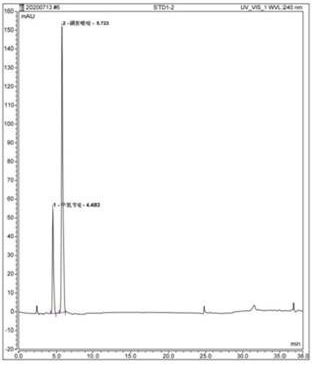
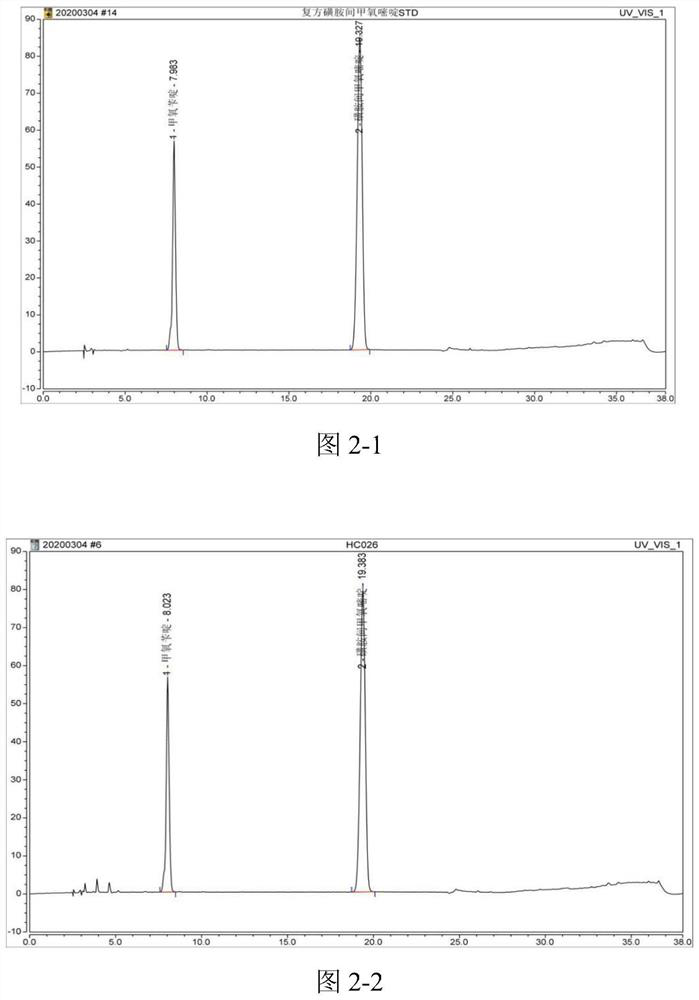
[0098] Preparation of standard stock solution Precisely weigh about 50mg of sulfadiazine reference substance and about 10mg of trimethoprim, put them in 50ml measuring bottles respectively, add an appropriate amount of methanol, sonicate for 10-20min, take it out, let it cool, dilute to the mark with methanol, Mix well to obtain a reference substance stock soluti...
Embodiment 2
[0105] The concrete method of content determination of compound sulfamethoxine injection comprises the following steps:
[0106] Adopt the same measuring method of embodiment 1, assay result:
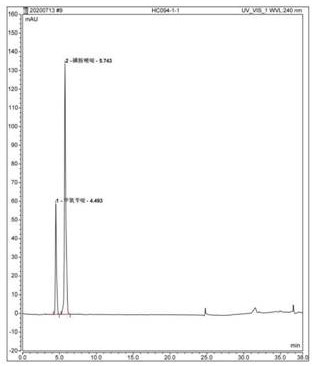
[0107] Accurately measure 10 μl each of the test solution or the reference substance working solution and inject it into the liquid chromatograph, and calculate the content by the peak area according to the external standard method. The contents of sulfamethoxine and trimethoprim in this batch of compound sulfamethoxine injection were measured to be 92.73% and 94.98% of the labeled amount respectively. The contents of sulfamethoxine and trimethoprim measured by the traditional method are respectively 99.0% and 98.1% of the marked amount, so it can be seen that the assay method of the present invention has high accuracy, good stability, more sensitive detection, environmental protection, and professional One and accurate, improve the safety and reliability of medication.
Embodiment 3
[0109] The concrete method of content determination of compound sulfamethoxine tablet comprises the following steps:
[0110] Adopt the same measuring method of embodiment 1, assay result:
[0111] Accurately measure 10 μl each of the test solution or the reference substance working solution and inject it into the liquid chromatograph, and calculate the content by the peak area according to the external standard method. The contents of sulfamethoxine and trimethoprim in the batch of compound sulfamethoxine tablets were measured to be 91.11% and 89.12% of the labeled amount respectively. The content of sulfamethoxine and trimethoprim measured by the traditional method is 92.36% and 91.45% of the labeled amount respectively. It can be seen that the assay method of the present invention has high accuracy, good stability, more sensitive detection, environmental protection and specificity. And accurate, improve the safety and reliability of medication.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com