Pyraclostrobin hapten, artificial antigen and antibody as well as preparation method and application thereof
A technology of pyraclostrobin and artificial antigen, which is applied in chemical instruments and methods, animal/human protein, serum albumin, etc., can solve the problem of incompatibilities in on-site detection, accurate detection and screening, inability to be widely used, and sample pretreatment cumbersome and other problems, to achieve the effect of easy control of reaction conditions, high purity and yield, and good affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
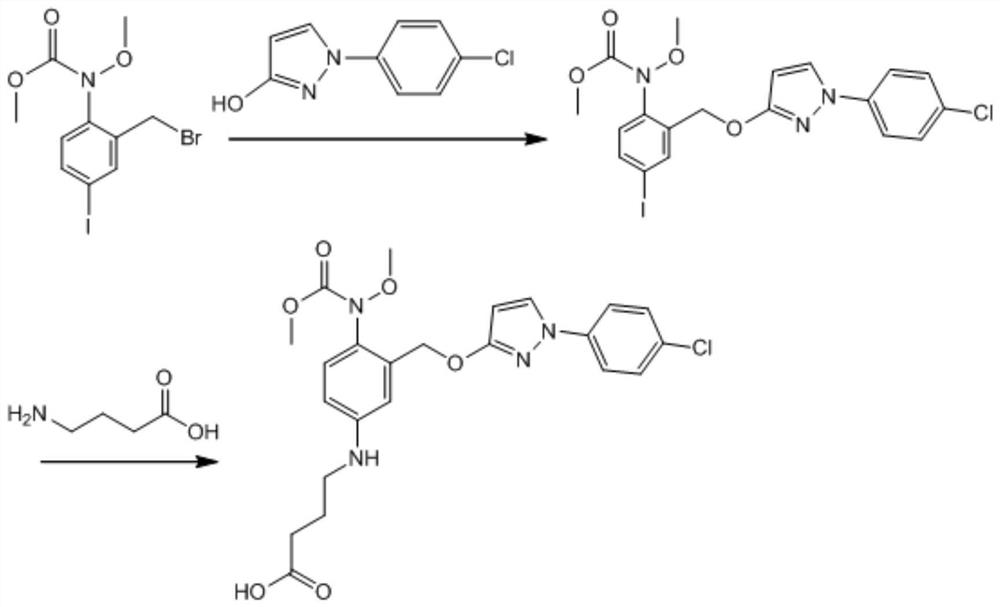
[0040] A preparation method of pyraclostrobin hapten, which comprises the steps of:
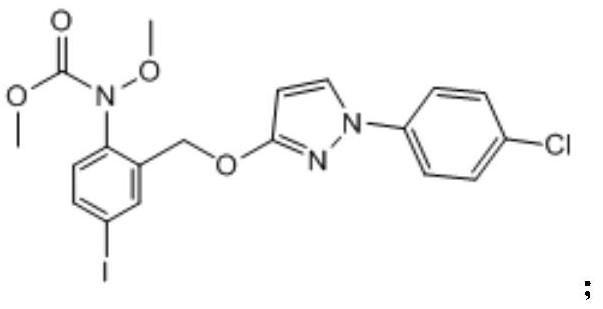
[0041] 1) Dissolve 4.0 g of methyl 2-(bromomethyl)-4-iodobenzene (methoxy) carbamate in 80 mL of N,N-dimethylformamide (DMF), add 1.12 g of KOH and 1.94g 1-(4-chlorophenyl)-2H-pyrazolin-3-one, after fully stirring, add 0.33g of anhydrous potassium iodide, heat and stir in an oil bath, react at 80°C for 7h, stop the reaction, cool to room temperature, Add 100mL of water and 200mL of ethyl acetate, oscillate, stand still, separate the water phase, add 80mL pure water to the organic phase, oscillate, stand still, separate the water phase, and evaporate the organic phase to dryness to obtain intermediate 1;
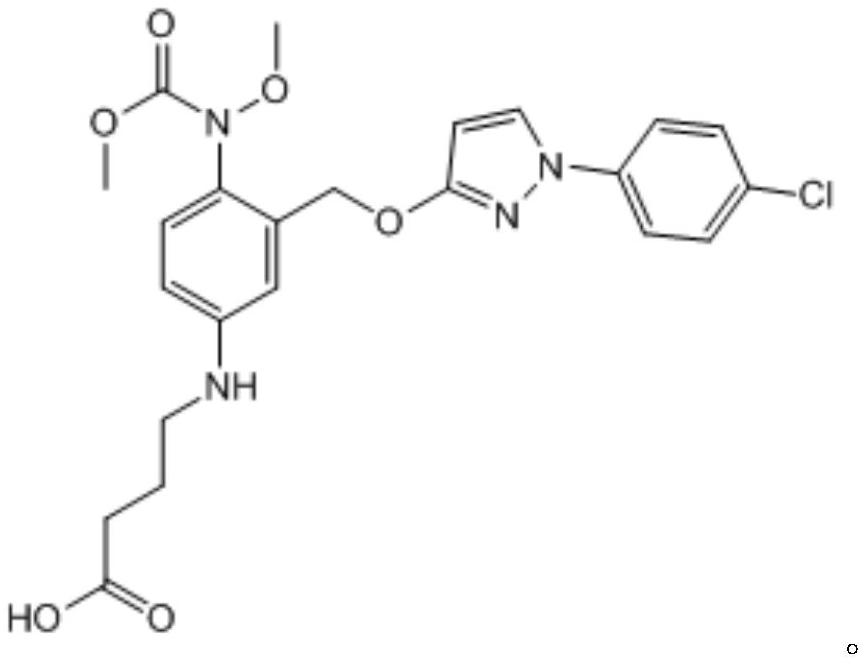
[0042] 2) Add 100mL dimethyl sulfoxide to dissolve all the intermediate 1, add 0.48g sodium hydride, 0.77g anhydrous cuprous iodide and 1.21g aminobutyric acid, stir well, react at 100°C for 9h, stop the reaction, and cool to room temperature, add 200mL water, adjust the pH to 5 with 6mol / L...
Embodiment 2
[0044] A preparation method of pyraclostrobin artificial antigen, the steps are as follows:
[0045]Take 17 mg of pyraclostrobin hapten prepared in Example 1, add 1 mL of DMF to dissolve, add 20 μL of triethylamine and 127 μL of isobutyl chloroformate, cool to 0-5 °C, and react for 3 hours to obtain hapten solution A; Take 50mg of bovine serum albumin (BSA), add 0.05mol / L PB buffer solution to dissolve to obtain liquid B; add liquid A dropwise to liquid B, react at 4°C for 12h, and dialyze and purify with 0.02mol / L PBS for 3 days. The medium was changed 3 times a day to obtain the pyraclostrobin artificial antigen coupled with bovine serum albumin, which was aliquoted and stored at -20°C.
Embodiment 3
[0047] A preparation method of pyraclostrobin artificial antigen, the steps are as follows:
[0048] Take 10 mg of the pyraclostrobin hapten prepared in Example 1, add 1 mL of 1,4-dioxane to dissolve, add 8.2 mg of N-hydroxysuccinimide (NHS), 1-(3-dimethylaminopropyl base)-3-Ethylcarbodiimide hydrochloride (EDC) 13mg, react at room temperature for 3h to obtain hapten solution A liquid; take ovalbumin (OVA) 50mg, add 0.05mol / L PB buffer solution to dissolve, and obtain Liquid B: Add liquid A dropwise to liquid B, react at 4°C for 12 hours, dialysis and purify with 0.02mol / L PBS for 3 days, change the liquid 3 times a day, and obtain pyraclostrobin artificial antigen coupled with ovalbumin , aliquoted, and stored at -20°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com