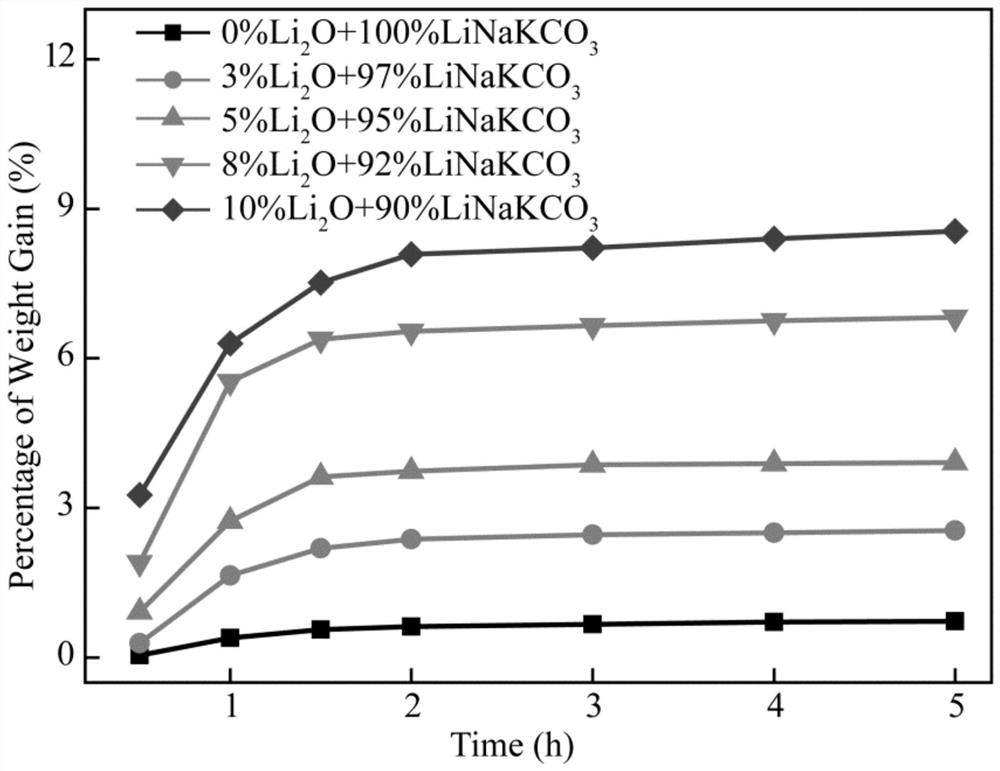
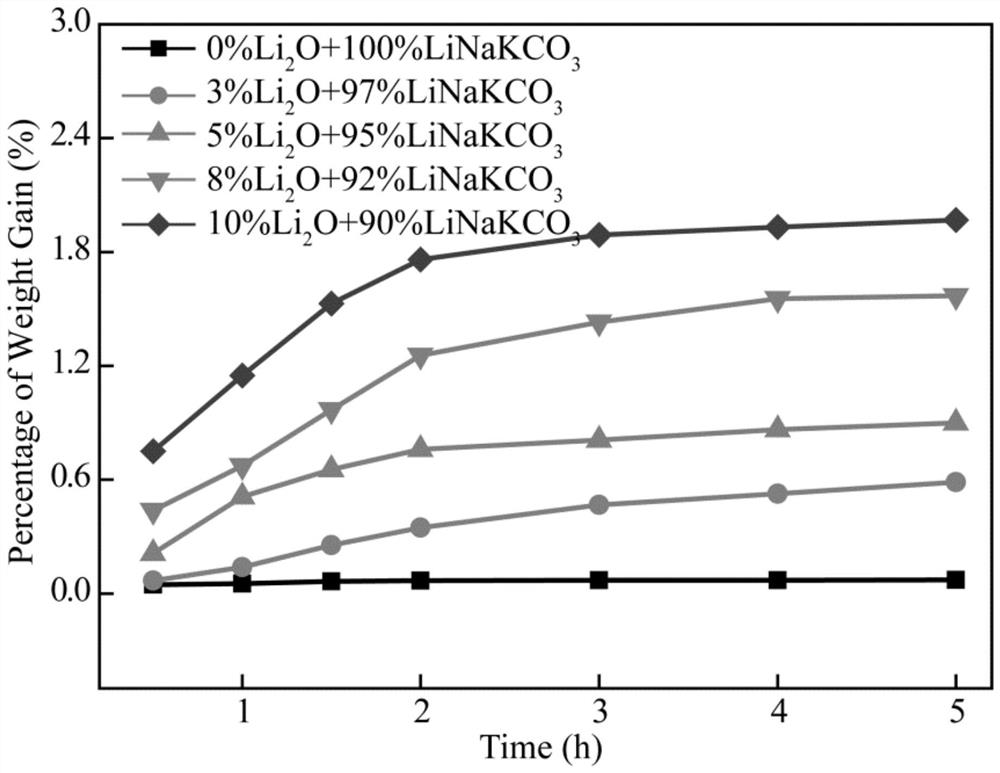
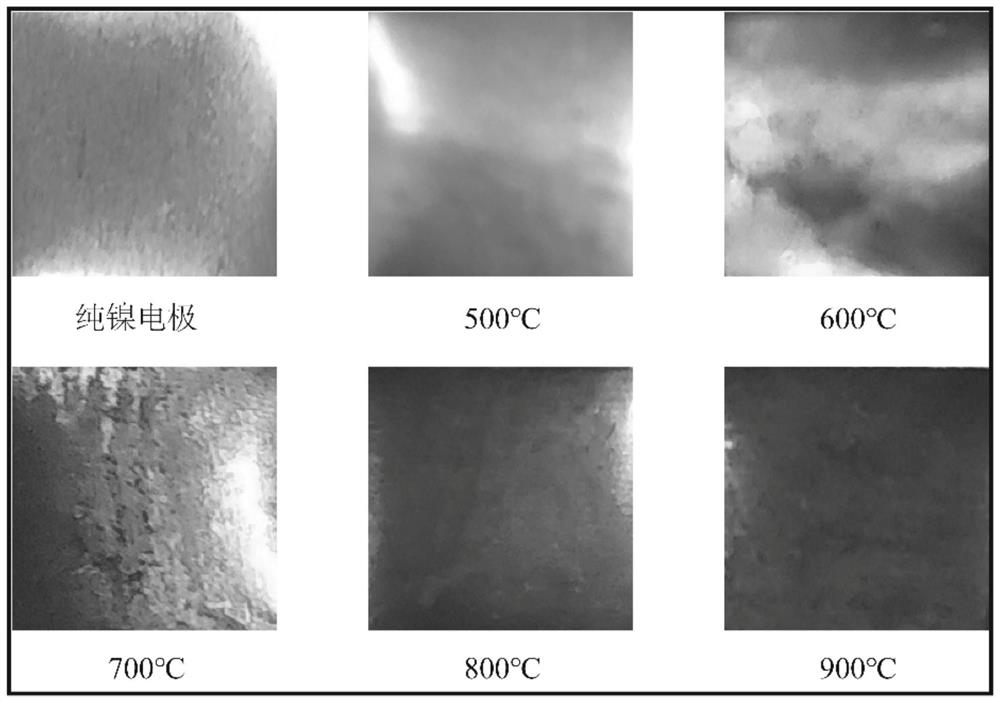
Method and system for preparing hydrocarbon-rich carbon-based fuel gas by driving carbon dioxide/water synergistic conversion through molten salt electrochemical method
A carbon dioxide, hydrocarbon-rich carbon-based technology, applied in electrodes, electrolysis process, electrolysis components, etc., can solve the problems of system short circuit, experiment failure, carbon powder falling off, etc., to improve corrosion resistance, avoid oxidation and corrosion, and prolong life. effect of time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: the corrosion resistance (durability) experiment of preoxidation film-forming nickel electrode
[0062] ① Na 2 CO 3 -K 2 CO 3 A total of 50g of mixed molten salt +0.2LiOH is put into an alumina crucible, the operating temperature is 700°C, and the surface area is 20cm 2 The nickel anode and nickel cathode were inserted into the molten salt medium; in an argon atmosphere, a 3h pre-oxidation film-forming treatment was performed at a constant voltage of 1.0V to obtain a molten salt in-situ pre-oxidation film-forming nickel anode.
[0063] ②Set the surface area to 20cm 2 The nickel electrode was heated in air at 800°C for 2 hours to prepare an air-heated pre-oxidized film-forming nickel anode.
[0064] The pre-oxidized film-forming nickel electrode prepared by the above two methods was used as the anode, and the iron with the same surface area was used as the cathode. 0.85 Na0.61 K 0.54 CO 3 +0.2LiOH mixed molten salt is used as the electrolyte, and th...
Embodiment 2
[0069] Example 2: H 2 Effect of O flow rate on gas product formation rate experiment
[0070] The surface area is 20cm 2 The pre-oxidized film-forming nickel anode and iron cathode obtained by the method of air heating film-forming in Example 1, at 575 ° C, the amount of electrolyte with a 2.0V constant voltage is 100g of Li 0.85 Na 0.61 K 0.54 CO 3 +0.2LiOH+0.05CaO molten salt system, the CO 2 The flow rate is controlled at 180mL / h, the Ar flow rate is controlled at 600mL / h, and the H 2 When the O flow rate is 0.10, 0.15, 0.20, 0.25g / h, the CH 4 and H 2 output rate, the result is as follows Figure 7 And as shown in Table 2: when H in the feed gas 2 When O flow rate is 0.10g / h, methane (CH 4 ) formation rate is 31.21mL / h, H 2 The formation rate reached 38.72mL / h, and the CH in the gas product 4 The content is low, and the economic value is not high; with the H in raw materials 2 Gradual increase of O flow rate, CH 4 The generation rate increases accordingly, whe...
Embodiment 3
[0072] Example 3: CO 2 Effect of flow rate on gas product generation rate experiment
[0073] The surface area is 20cm 2 The pre-oxidized film-forming nickel anode and iron cathode obtained by the method of air heating film-forming in Example 1, at 575 ° C, the amount of electrolyte with a 2.0V constant voltage is 100g of Li 0.85 Na 0.61 K 0.54 CO 3 +0.2LiOH+0.05CaO molten salt system, H 2 The flow rate of O is controlled at 0.15g / h, and the flow rate of Ar is controlled at 600mL / h. 2 When the flow rate is 180, 300, 600, 900mL / h, the CH in the gas product 4 and H 2 output rate, the result is as follows Figure 9 and Table 2: When CO 2 When the flow rate is 180mL / h, CH 4 The production rate was the highest, reaching 34.66mL / h. But with CO 2 As the flow rate increases, the CO inside the reactor 2 As the partial pressure increases, the whole system tends to undergo elemental carbon deposition reaction, making CH in the product 4 Spawn rate decreased. Due to the ele...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com