Method for universally preparing high-entropy alloy nanoparticles
A nanoparticle and high-entropy alloy technology, applied in nanotechnology, nanotechnology, chemical instruments and methods, etc., can solve the problems of large differences in physical and chemical properties of metals, loss of active components, complex preparation process, etc., to achieve carrier selection The effect of wide range, increased dispersion, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
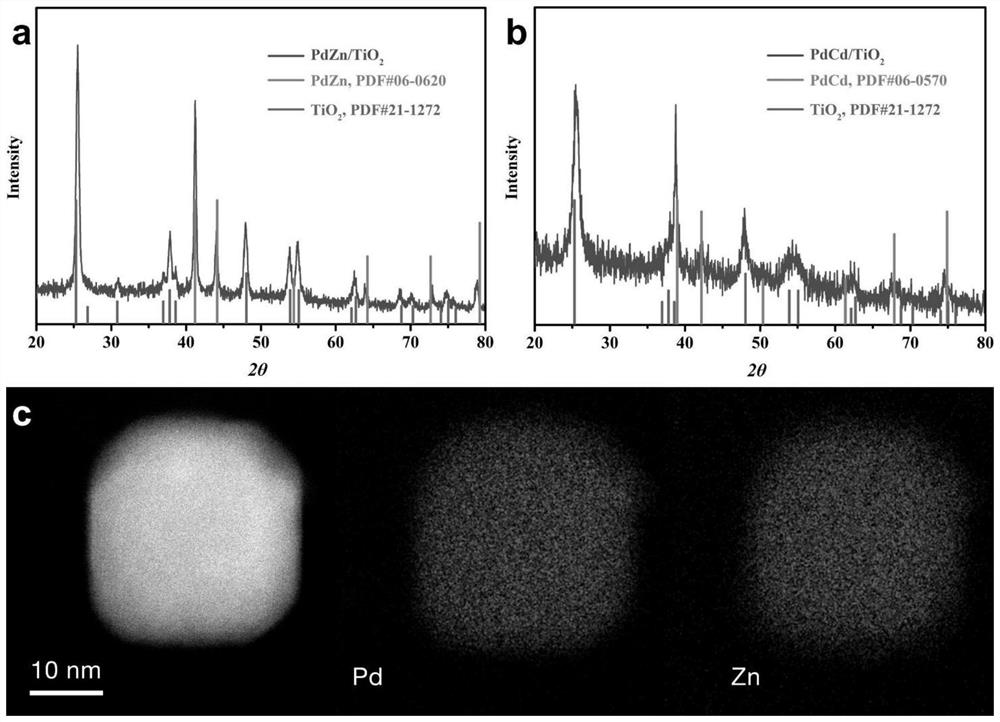
[0039] Binary Alloy PdZn / TiO 2 and PdCd / TiO 2 The synthesis of catalyst, the steps are as follows:
[0040] (1) Prepare a metal organic salt solution: dissolve palladium acetylacetonate and zinc acetylacetonate (cadmium) in a small amount (0.5-2 mL) of chloroform according to an equiatomic ratio, and sonicate for 10 minutes.
[0041] (2) Prepare the carrier dispersion: Weigh a certain mass of titanium dioxide (the alloy loading is 0.5-20wt.%) and add it into 5-30mL chloroform for ultrasonic dispersion for 1-5h to obtain the carrier dispersion.
[0042] (3) Add the metal organic salt solution to the carrier dispersion liquid, and ultrasonically disperse for 0.5h-2h. Under the condition of magnetic stirring, volatilize the solvent on a heating plate at 80°C to obtain a solid, then add a little chloroform (about 0.5mL) to infiltrate the solid, without obvious liquid flow, sonicate for 2-10min, heat and volatilize without stirring , redispersing the metal organic salt on the su...
Embodiment 2
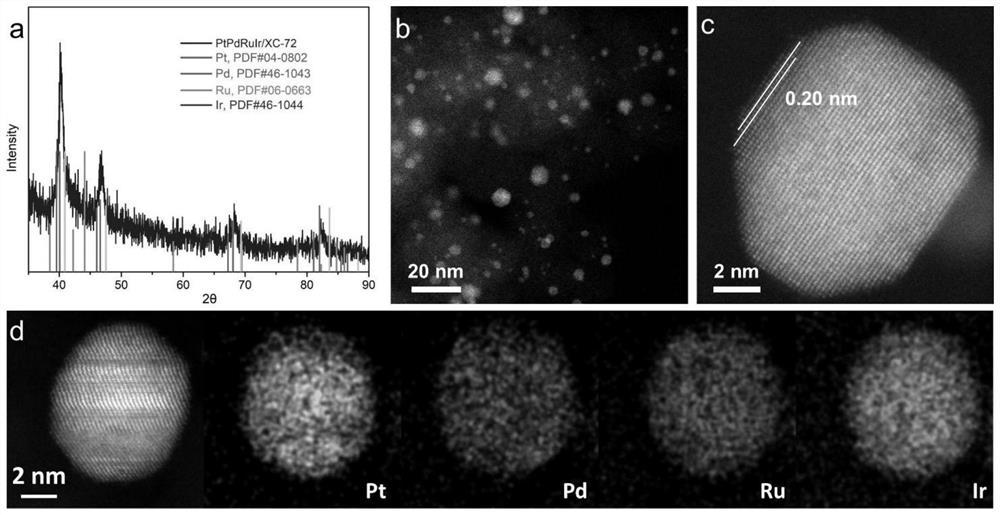
[0045] The synthesis of binary or higher alloys and high-entropy alloy nanoparticles is as follows:
[0046] (1) Configure metal organic salt solution: take more than two kinds of platinum acetylacetonate, palladium acetylacetonate, ruthenium acetylacetonate, rhodium acetylacetonate, iridium acetylacetonate, iron acetylacetonate (Ⅱ / Ⅲ), cobalt acetylacetonate (Ⅱ / Ⅲ) ), nickel (II) acetylacetonate, (triphenylphosphine) gold chloride (I), copper acetylacetonate, manganese acetylacetonate, zinc acetylacetonate, bismuth neododecanoate, cadmium acetylacetonate, chromium acetylacetonate, dichloro Metal organic salts such as dimethyl germanium, tin (II) acetylacetonate, aluminum acetylacetonate, indium acetylacetonate, gallium acetylacetonate, vanadium acetylacetonate, molybdenum acetylacetonate, lead acetylacetonate, etc., can be dissolved in a small amount (0.5- 5mL) chloroform, the concentration of the metal organic salt was 1mmol / mL, and sonicated for 10min.
[0047] (2) Prepare t...
Embodiment 3
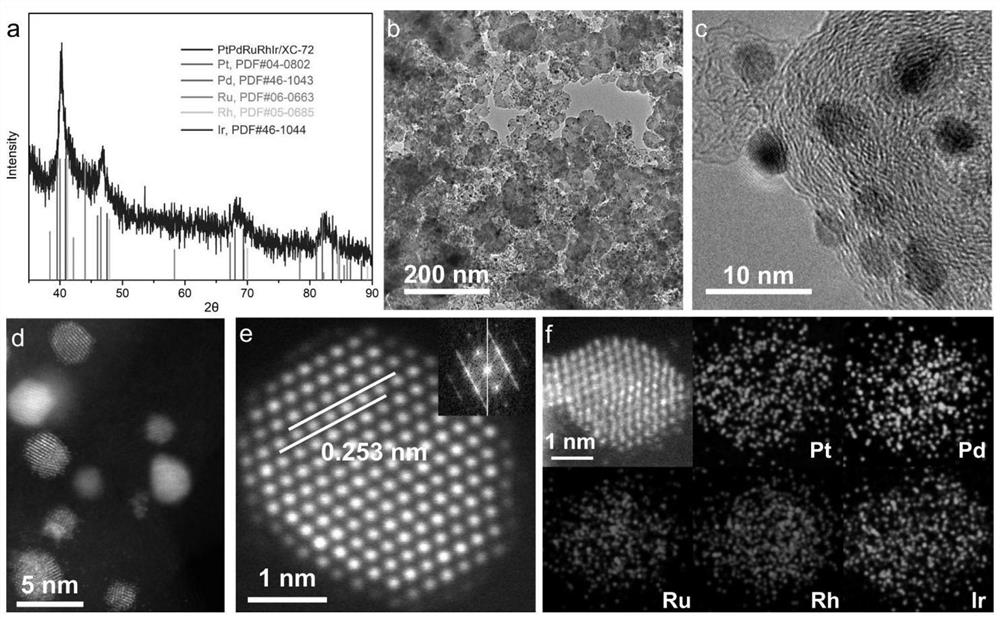
[0051] With reference to the method described in Example 2, the metal organic salt in this example is platinum acetylacetonate, palladium acetylacetonate, ruthenium acetylacetonate, rhodium acetylacetonate, iridium acetylacetonate, other conditions are the same as in Example 2, and the obtained PtPdRuRhIr / XC- 72 high-entropy alloy nanoparticles, image 3 It is the structure characterization and element distribution map of PtPdRuRhIr / XC-72.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com