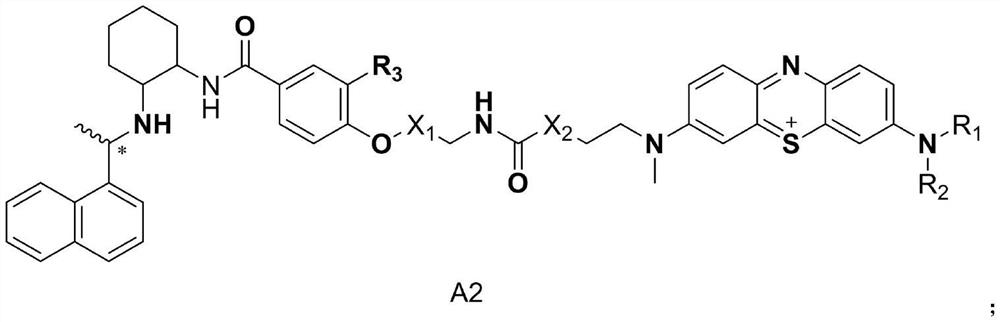
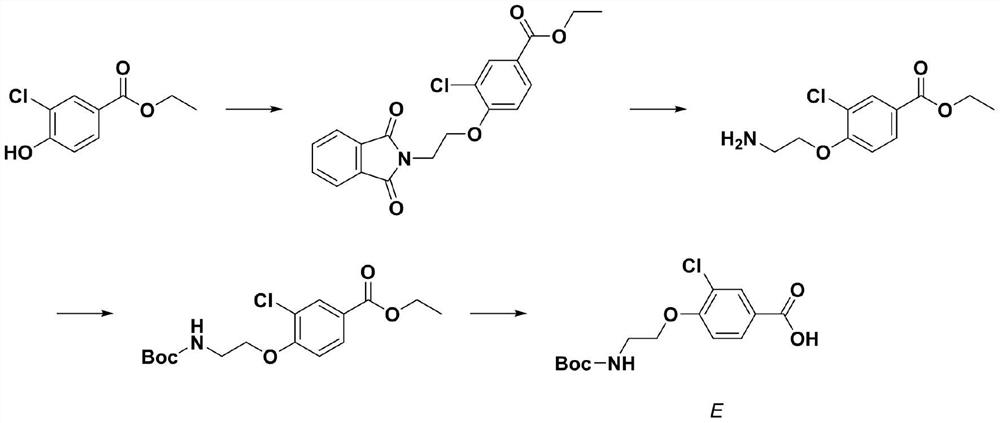
Molecular probe derived from calcium-sensitive receptor protein antagonist and application of molecular probe in parathyroid gland resection surgery
A technology of molecular probes and calcium sensitive proteins, applied in the field of molecular probes, can solve the problems of large number, high failure rate, and small tissue, and achieve the effects of small molecular weight, high safety, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
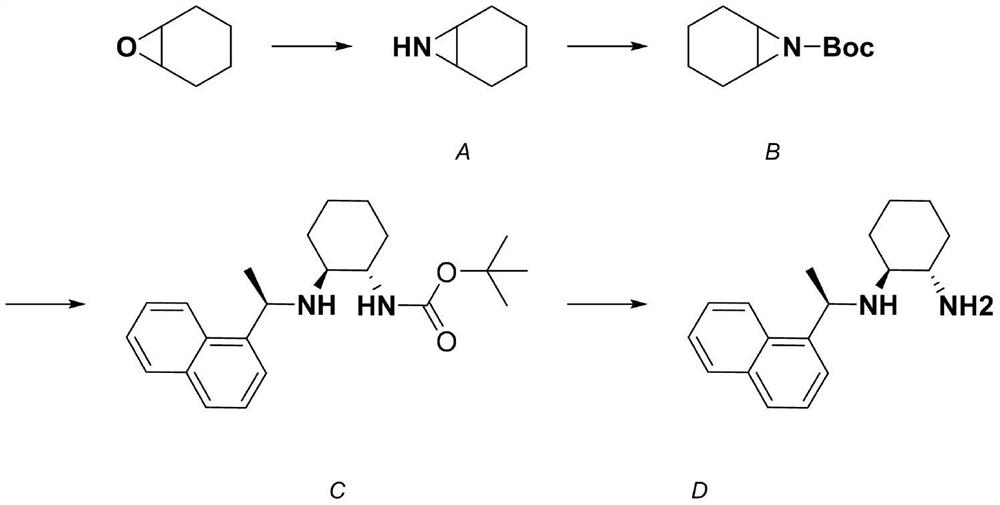
Embodiment 1
[0050] Preparation of Compound A
[0051] 2-Azidocyclohexan-1-ol ((1R,2R)-2-azidocyclohexan-1-ol) synthesis procedure: epoxycyclohexane (15.5mL, 153mmol) and NaN 3 (25.2g, 387mmol) dissolved in H 2 In a mixed solution of O and acetone (1:1, 160 mL), heat to reflux for 17 hours (the reaction temperature is set to 75° C.). After the reaction was completed, the acetone was evaporated away, and the remaining aqueous system was extracted with ether (3*80mL). The combined extracts were washed twice with water (2*20mL) and then washed with MgSO 4 dry. The solvent was evaporated to dryness to obtain 2-azidocyclohexanol as a yellow oil (yield 99%), which was directly used in the next synthesis reaction. Rf0.6 (petroleum ether: ethyl acetate, 9:1). 1HNMR (500MHz, Chloroform-d) δ3.41(q, J=6.9Hz, 1H), 2.04(q, J=6.9Hz, 1H), 1.97(s, 1H), 2.01–1.89(m, 1H), 1.77–1.67(m,1H),1.71–1.61(m,2H),1.64–1.57(m,1H),1.52–1.40(m,1H),1.39(dt,J=12.6,6.7Hz,1H), 1.32 (dt, J = 12.5, 6.9 Hz, 1H). 13C NMR...
Embodiment 2
[0055] Preparation of Compound B
[0056] Boc-7-azabicyclo[4.1.0]heptane-7-carboxylic acid tert-butyl ester (tert-butyl 7-azabicyclo[4.1.0]heptane-7-carboxylate) B compound synthesis: under nitrogen atmosphere, Compound A (0.194g, 2mmol), triethylamine (0.525g, 5.2mmol) and a catalytic amount of 4-dimethylaminopyridine (DMAP) were dissolved in 3mL of anhydrous dichloromethane, and the solution was cooled to 0°C and then added with di Di-tert-butyl carbonate (0.905 g, 5.2 mmol). After the solution was stirred at 0°C for 10 minutes, the reaction was stirred at room temperature for an additional 2 hours. After the reaction was completed, the reaction was quenched with 20 mL of water. This system was diluted with saturated ammonium chloride solution (20 mL). The aqueous phase was extracted with ether (2*25mL) and the combined extracts were washed with saturated KHSO 4 (20mL) solution, saturated Na 2 CO 3 (20 mL) and saturated NaCl (20 mL), the organic phase was then dried. ...
Embodiment 3
[0058] Synthesis of compound C (tert-butyl (2-(((S)-1-(naphthalen-1-yl)ethyl)amino)cyclohexyl)carbamate):
[0059] Compound B (1 mmol) and LiClO 4 (0.1 mmol) was dissolved in anhydrous acetonitrile (6 mL), and (S)-(-)-1-(1-naphthyl)ethylamine (1.25 mmol) was added to the solution under an inert atmosphere at room temperature. The reaction solution was refluxed until the reaction was complete (check the reaction with TLC, it takes about 4-8 hours). After the reaction system was spun to remove most of the acetonitrile, the crude product was distributed in a two-phase system of ethyl acetate and water, and the organic phase was washed with water and brine, then dried with anhydrous sodium sulfate, concentrated and then used as ethyl acetate and petroleum ether. The pure product was separated by mobile phase chromatography column. There are two configurations, SSR and RRR, which are separated by silica gel chromatography (dichloromethane: methanol volume ratio = 100: 10-20), and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com