Multi-block polyether type macromolecular surfactant as well as preparation method and application thereof
A technology of surfactant and block polyether, which is applied in chemical instruments and methods, dyeing methods, textiles and papermaking, etc., can solve the problems of disordered detonation, accelerated reaction speed, temperature rise, etc., and achieves the goal of reducing detonation The effect of risk, smooth reactivity, and good dispersion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
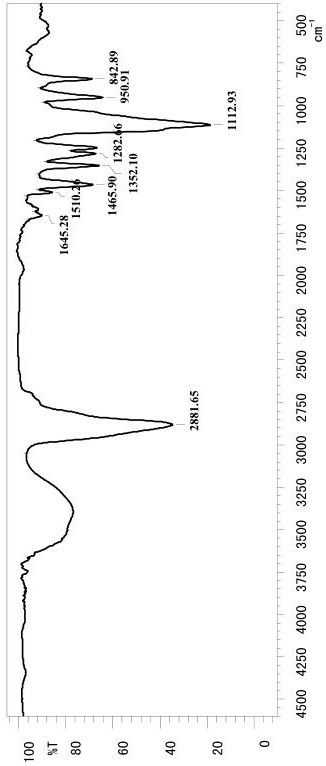
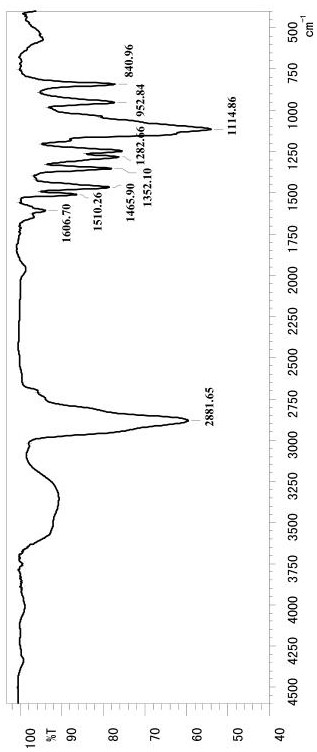
Image
Examples
preparation example Construction
[0083] The present invention also discloses a preparation method of the surfactant of the above molecular structural formula (1), comprising the following steps:
[0084] (1) Add the monofunctional raw material with a single active hydrogen and the bifunctional raw material with two active hydrogens into the high-pressure alkoxylation reactor at a molar ratio of 1:0.5 to 10, and then put them into the The catalyst I containing 0.02-1% of the total mass of monofunctional raw materials and bifunctional raw materials is heated to 70-130°C and vacuumed for 30-90 minutes to remove the generated water or small organic molecules, so that the raw materials in the reactor contain The amount of water is less than 0.1% of the raw material mass, and the reaction system is replaced with nitrogen, so that the oxygen content in the reactor is lower than 500ppm. After the nitrogen replacement is completed, 1,2-epoxyalkylene compounds are introduced to carry out multi-stage block or mixed poly...
Embodiment 1
[0104] Put 150 grams (about 0.5mol) cardanol, 142 grams (about 0.45mol) cardol, 16.5 grams (about 0.05mol) methyl cardol into the autoclave, then add 1.54 grams of potassium hydroxide powder, Raise the temperature to 110°C for dehydration for 60 minutes, take a sample to detect that the moisture in the material is less than 0.1%; replace the nitrogen, and after the oxygen content in the kettle is lower than 500ppm, continue to add 264 grams (about 6mol) of ethylene oxide, and control the reaction temperature at 155- Between 165°C, the introduction of ethylene oxide is completed; then continue to add 261 grams (about 4.5mol) of propylene oxide, control the reaction temperature at 125-135°C, and the introduction of propylene oxide is completed; then continue to add 792 grams (about 18mol) ) Ethylene oxide, control the reaction temperature at 155-165°C, after the ethylene oxide is thrown in, keep warm at 120-165°C, and mature for 2 hours to ensure that the terminal of the generate...
Embodiment 2
[0111]Put 150 grams (about 0.5mol) of cardanol, 142 grams (about 0.45mol) of cardol, and 16.5 grams (about 0.05mol) of 2-methylcardol into the autoclave, and then add 4.9 grams of 30% methanol Sodium methanol solution, heated to 100°C for 60 minutes to demethanol, sampling and testing the moisture in the material is less than 0.1%. Replace nitrogen, after the oxygen content in the kettle is lower than 500ppm, continue to add 2640 grams (about 60mol) of ethylene oxide, control the reaction temperature at 155-165°C, after the introduction of ethylene oxide is complete, keep warm at 120-165°C, and mature for 30 minutes , until the reactor pressure no longer changes, that is, the unreacted ethylene oxide is basically exhausted. Lower the temperature to 100°C, vacuumize and degas for 0.5 hours to remove unreacted ethylene oxide and other non-condensable gases. Cool down to 80°C, add 1.63 g of acetic acid, and stir for 30 minutes. Transfer the above reaction product to a polymeriz...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com