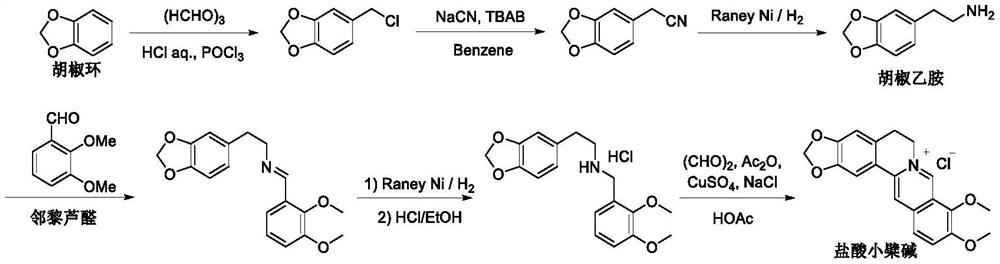
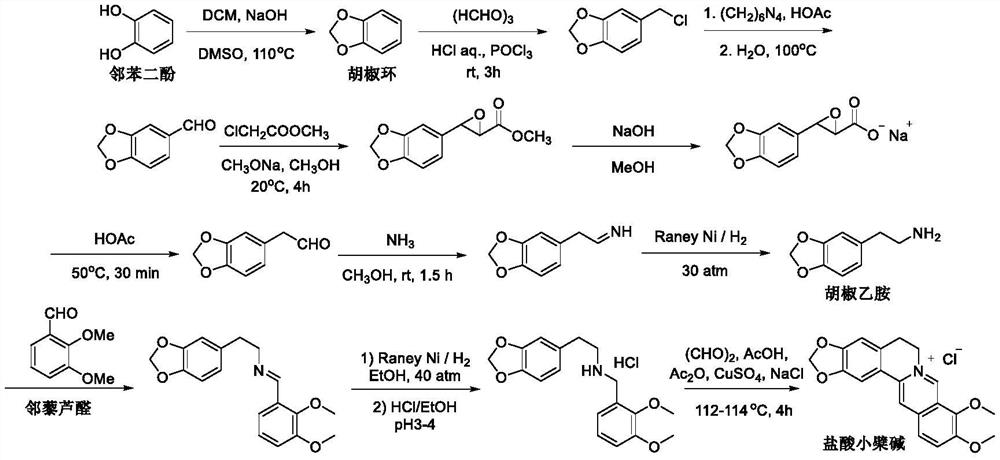
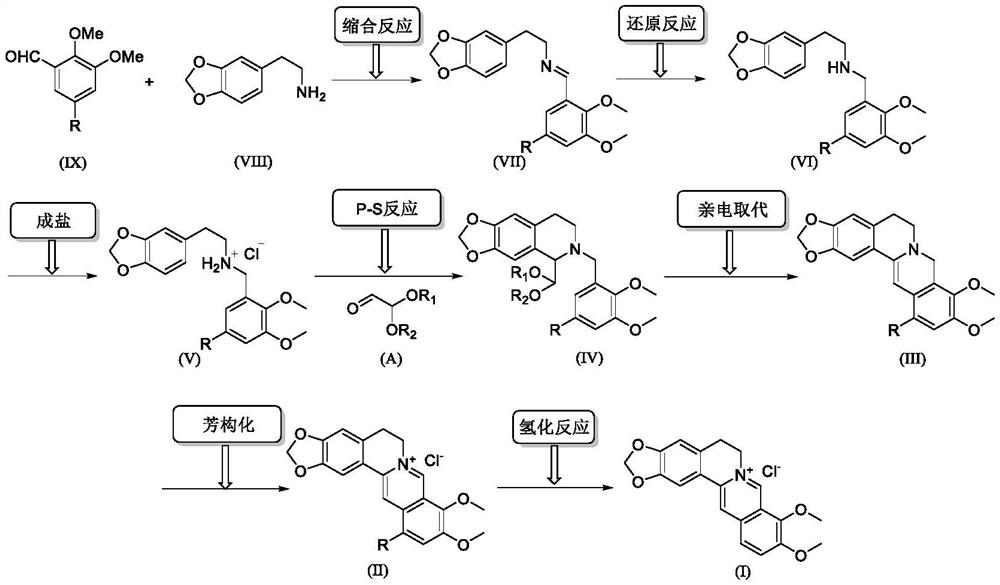
A kind of synthetic preparation method of berberine hydrochloride
A technology of berberine hydrochloride and a synthesis method, applied in the field of organic chemistry, can solve the problems of increased treatment cost of copper-containing wastewater, cumbersome synthesis steps, complicated operation, etc., and achieves reduction of labor protection cost, high chemical yield, and mild reaction conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0165] Example 1 Preparation of 4-bromoguaiacol
[0166] Under argon protection, guaiacol (25 g, 201 mmol) was uniformly dispersed in 125 mL of chloroform, and then the reaction solution was pre-cooled at -5 °C for 10 minutes, and bromine (10.3 ml, 201 mmol) was slowly added dropwise thereto. chloroform (75mL) solution, try to keep the system colorless. After the dripping was completed, the reaction was moved to room temperature for 1 hour, and the reaction was detected by TLC dot plate. With 150mL Sat.NaHSO 3 (aq) Quenching the reaction, separating the liquids in a separating funnel, extracting the aqueous layer three times with chloroform, combining the organic phases, backwashing once with saturated brine, drying over anhydrous sodium sulfate and spin-dried to obtain pale yellow crystals Crude. Under reduced pressure distillation (97-100°C / 2mmHg, Lit. 119-120°C / 5mmHg), 4-bromoguaiacol (40g, 94%, mp=31-32°C) was obtained.
[0167] 1 H NMR (400MHz, Chloroform-d) δ 6.99 (...
Embodiment 2
[0168] Example 2 Preparation of 2-hydroxy-3-methoxy-5-bromomandelic acid
[0169] Under ice bath, 4-bromoguaiacol (2 g, 9.8 mmol) was gradually added dropwise to a solution of sodium hydroxide (692 mg, 17.3 mmol) in water (12 mL), and then alumina powder (441.3 mmol) was gradually added thereto. mg, 4.33 mmol). After stirring for 5 minutes in an ice bath, an aqueous solution of glyoxylic acid (50% wt in H 2 O, 1.54g, 10.4mmol), the pH value of the reaction solution is 9-10. The reaction was then moved to a 60°C oil bath for 6 hours, and the reaction was monitored by TLC. After the reaction solution was cooled to room temperature, the alumina powder was removed by suction filtration and the filter cake was washed with a small amount of 20% sodium hydroxide. The filtrate was collected, and the pH value of the filtrate was adjusted to 3-4 with concentrated hydrochloric acid, and the aqueous layer was extracted with toluene 4 times. 63%). The aqueous layer was then adjusted t...
Embodiment 3
[0171] Example 3 Preparation of 2-hydroxy-3-methoxy-5-bromobenzaldehyde
[0172] At room temperature, 25 mL aqueous solution of crude 2-hydroxy-3-methoxy-5-bromomandelic acid (5 g, 18 mmol) was fully mixed with 100 mL of toluene, then moved to 90 °C oil bath for heating, and then slowly added 20 mL of water to the reaction system. % Aqueous solution of ferric chloride (22 mL, 27 mmol), after the dripping was completed, the reaction was continued by heating in an oil bath at 90° C. for 4 hours, and the reaction was monitored by TLC. After the reaction solution was cooled to room temperature, an appropriate amount of toluene was added to the reaction system to dilute, and the reaction solution was extracted with toluene in a separating funnel for 4 times, and the organic phases were combined. 2 SO 4 After drying, spin-dried to obtain 2-hydroxy-3-methoxy-5-bromobenzaldehyde (2.4 g, 85%, mp=115-116° C.) as a pale yellow solid. The aqueous layer was further adjusted to pH 1 with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com