Efficient nickel-based self-assembly oxygen evolution electrode
An oxygen evolution electrode and self-assembly technology, which is applied in the direction of electrodes, electrolysis process, electrolysis components, etc., can solve the problems of weak bonding method, harsh preparation conditions, and affecting the life of electrodes, etc., to achieve lower energy barriers, large active specific surface area, The effect of low overpotential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] The first step: Ultrasonic the nickel mesh in ethanol and acetone solutions for 10 minutes to remove the surface grease layer; then ultrasonically in the hydrochloric acid solution with a concentration of 3 moles per liter for 10 minutes to remove the surface oxide layer.
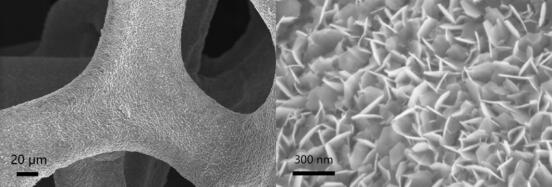
[0022] The second step: Add 0.16 grams of sodium hydroxide, 1 milliliter of hydrogen peroxide and 50 milliliters of deionized water into a 100 milliliter hydrothermal kettle, and then put the nickel mesh treated in the first step together In a hydrothermal kettle, react with 150 degrees centigrade hydrothermally for 6 hours, after the reaction finishes, wash and dry.
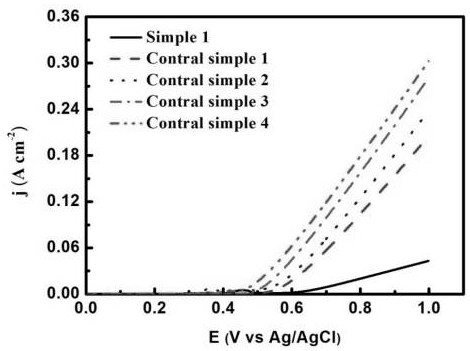
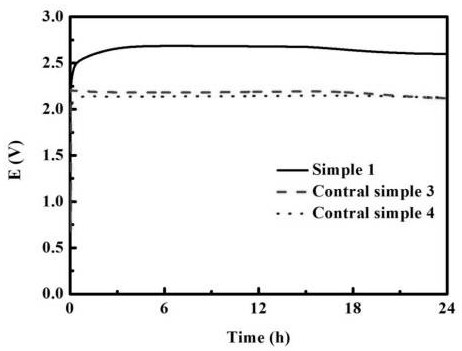
[0023] Shanghai Chenhua CHI 660E electrochemical workstation was used to test the oxygen evolution performance and life performance of the electrode. The performance test adopts a three-electrode system for testing. The hydrothermally treated nickel mesh electrode is used as the working electrode, the silver / silver chloride electrode i...
Embodiment 2
[0025] Step 1: Sonicate nickel foam in ethanol and acetone solutions for 10 minutes each to remove the surface grease layer; then sonicate in hydrochloric acid solution with a concentration of 3 moles per liter for 10 minutes to remove the surface oxide layer.
[0026] The second step: Add 0.16 grams of sodium hydroxide, 1 milliliter of hydrogen peroxide and 50 milliliters of deionized water into a hydrothermal kettle with a volume of 100 milliliters, and then put the nickel foam treated in the first step together In a hydrothermal kettle, react with 150 degrees centigrade hydrothermally for 6 hours, after the reaction finishes, wash and dry.
[0027] Shanghai Chenhua CHI 660E electrochemical workstation was used to test the oxygen evolution performance and life performance of the electrode. The performance test adopts a three-electrode system for testing. The hydrothermally treated foamed nickel electrode is used as the working electrode, the silver / silver chloride electrode ...
Embodiment 3
[0029] The first step: Ultrasonic the nickel mesh in ethanol and acetone solutions for 10 minutes to remove the surface grease layer; then ultrasonically in the hydrochloric acid solution with a concentration of 3 moles per liter for 10 minutes to remove the surface oxide layer.
[0030] The second step: Add 0.16 grams of sodium hydroxide, 1 milliliter of hydrogen peroxide and 50 milliliters of deionized water into a 100 milliliter hydrothermal kettle, and then put the nickel mesh treated in the first step together In a hydrothermal kettle, react with 150 degrees centigrade hydrothermally for 6 hours, after finishing, wash and dry.
[0031] The third step: the electrode obtained in the previous step is used as the anode, and the conductive hydrophilic carbon cloth is used as the cathode. In the ferrous ammonium sulfate solution of 10 millimoles per liter, the chronopotentiometry is adopted, and the current density of 1.2 milliamperes per square centimeter is used. Polarization...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com