Thiadiazolobenzotriazole near-infrared organic photoelectric small molecule as well as preparation method and application thereof
A benzotriazole and near-infrared technology, used in photovoltaic power generation, organic chemistry, circuits, etc., can solve problems such as the lack of organic optoelectronic materials, and achieve the effects of good application prospects, good solubility and high crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
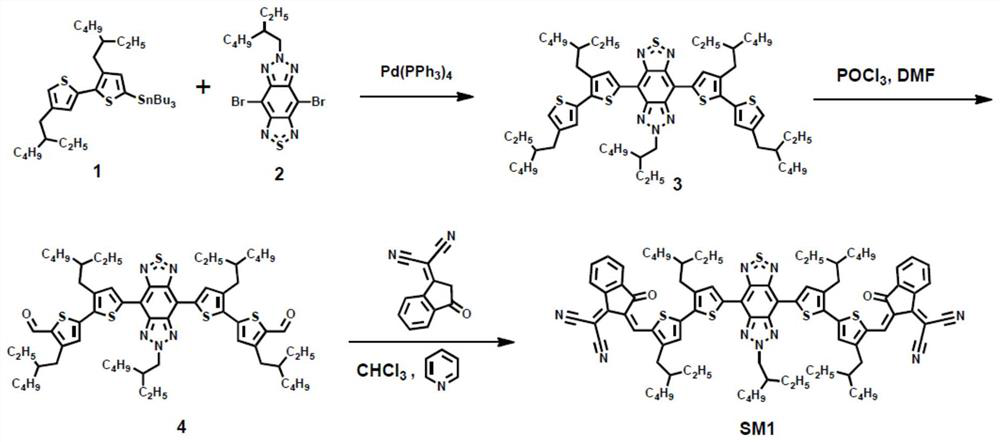
[0036] Synthesis of embodiment 1, SM1.
[0037] chemical reaction flow chart figure 1 Shown, concrete reaction steps and reaction conditions are as follows:
[0038] Compound 3 ( figure 1 The preparation of the compound marked as 3): under an inert atmosphere, compound 1 (680mg, 1mmol) and compound 2 (112mg, 0.25mmol) in anhydrous toluene (10ml) solution, add Pd (PPh 3 ) 4 (11.6 mg, 0.01 mmol). The whole reaction system was heated to 110°C and stirred for 36 hours. After the reaction solution was cooled, methanol was added for precipitation, centrifuged, and the solid part was dissolved with chloroform, washed three times with water, and dried over anhydrous magnesium sulfate. The organic phase was rotary evaporated to remove the solvent, using petroleum ether:dichloromethane (20:1) as eluent, separated by silica gel column chromatography, and the solvent was removed by rotary evaporation to obtain compound 3 (149 mg, 56%), MS (MALDI- TOF): m / z=1066.8.
[0039] Compound...
Embodiment 2
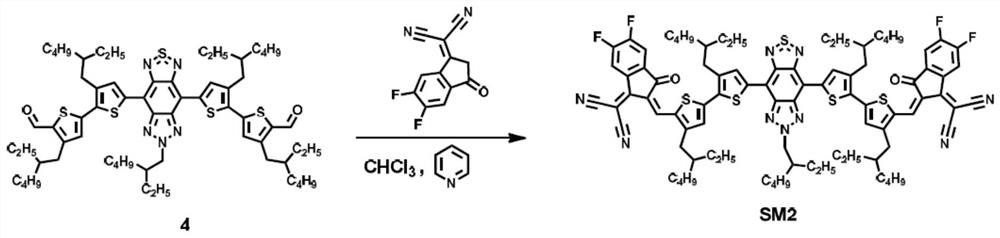
[0041] Synthesis of embodiment 2, SM2.
[0042] Wherein the synthesis of compound 3 and 4 is the same as in Example 1, and the chemical reaction flow diagram is as follows figure 1 Shown, the preparation reaction steps and reaction conditions of compound SM2 are as follows:
[0043] Under an inert atmosphere, 2-(5,6-difluoro-3-oxo-2,3-dihydro-1H-inden-1-ylidene)malononitrile (115 mg, 0.5 mmol) was added to compound 4 (56 mg , 0.05mmol) and pyridine (0.2mL, 2.5mmol) in anhydrous chloroform (15ml), heated to 60°C and stirred for 12 hours. After the reaction liquid was cooled down, methanol was added for precipitation, centrifuged, and the solid part was dissolved with chloroform, washed with water three times, and dried over anhydrous magnesium sulfate. The solvent was removed by rotary evaporation of the organic phase, petroleum ether: chloroform (2:1) was used as eluent, and the product was separated by silica gel chromatography. The product was recrystallized from chlorofo...
Embodiment 3
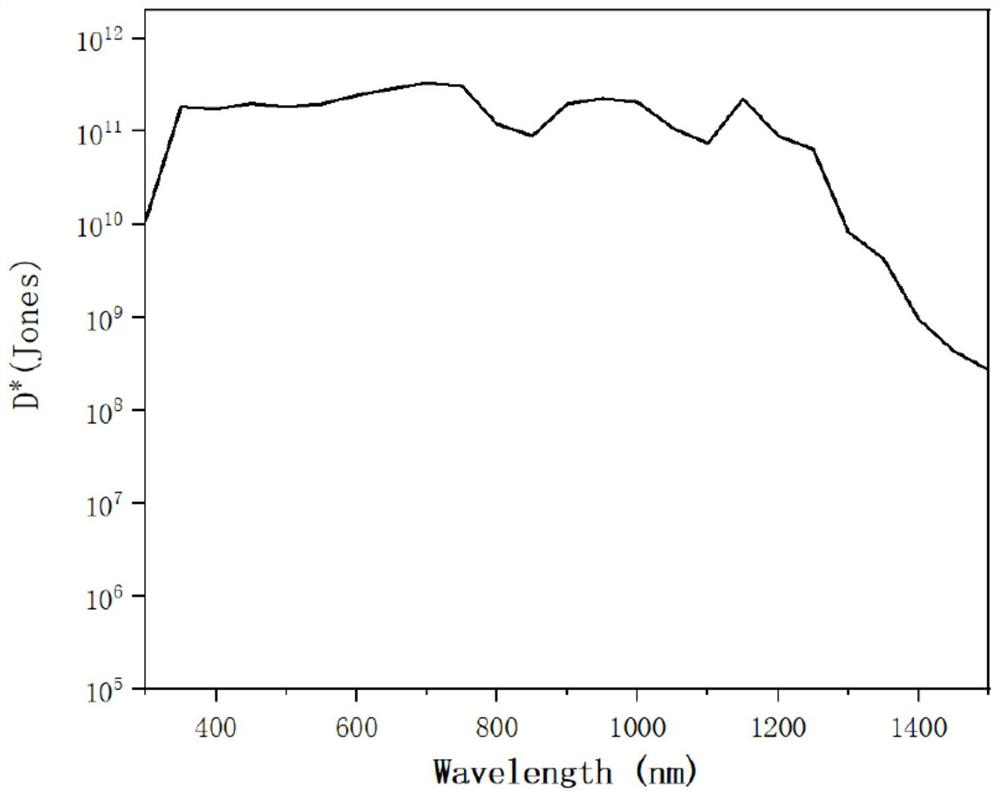
[0044] Example 3, Determining the photodetection performance test of organic optoelectronic molecules SM1 and SM2
[0045] Diode-type organic photodetection devices were prepared by solution spin coating with thiadiazolobenzotriazole near-infrared organic optoelectronic small molecules SM1 and SM2 prepared in Examples 1 and 2 as acceptor materials and PTB7-Th as donor materials. The device structure is ITO / ZnO / SM1 or SM2:PTB7-Th / MoOx / Ag.
[0046] The preparation method is as follows: the transparent conductive glass with ITO is ultrasonically cleaned with deionized water, acetone, and isopropanol successively for 15 minutes, and then the surface of the substrate is treated with ozone. A 30nm thick ZnO modification layer is coated on the surface of ITO. The organic optoelectronic molecules SM1 and SM2 were blended with PTB7-Th (1:1 mass ratio) and dissolved in chlorobenzene to obtain a 40 mg / mL solution. The solution is uniformly spin-coated on the ZnO modification layer at a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com