Zinc-air cell electro-catalyst and preparing method thereof
A zinc-air battery and electrocatalyst technology, which is applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of short working life, complicated manufacturing process, and low catalytic activity, and achieve quality and High production efficiency, high electrocatalytic activity, and the effect of high electrocatalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
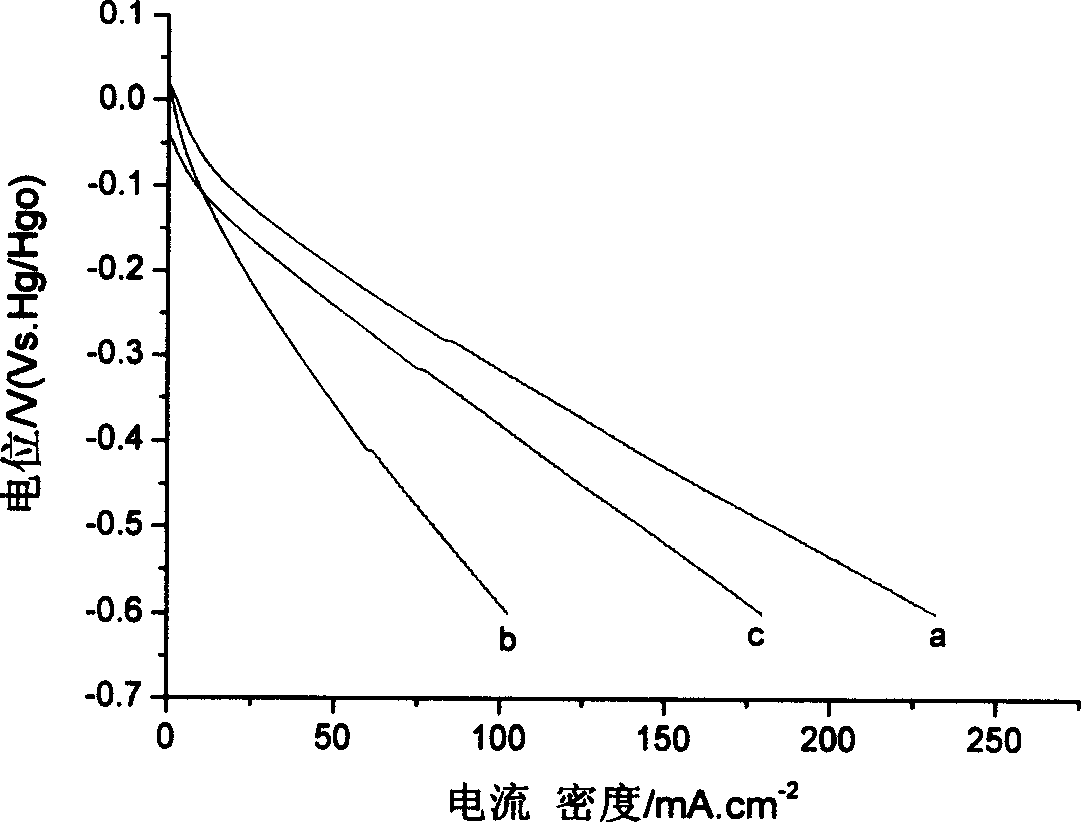
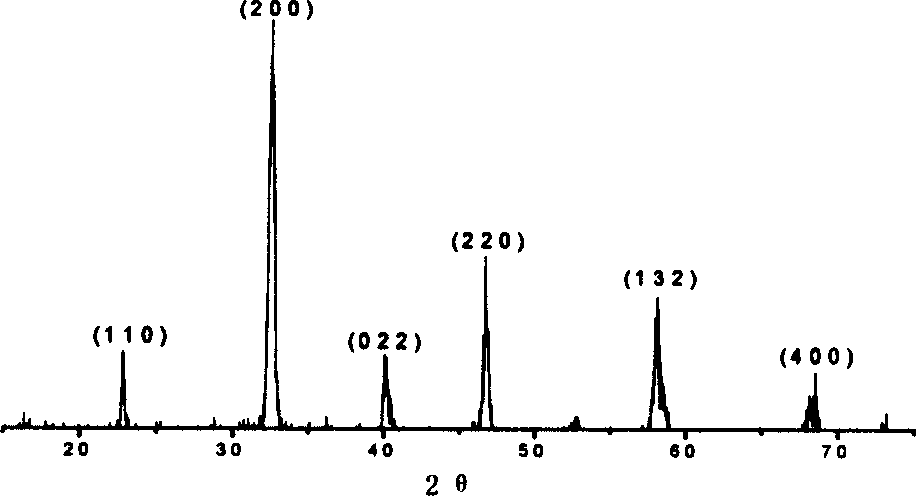
Image
Examples
Embodiment 1
[0031] Preparation of electrocatalyst: La(NO 3 ) 3 ·6H 2 O and Mn(CH 3 COO) 2 , add deionized water to make a saturated aqueous solution, then add tartaric acid and ethylene glycol in the above reaction system by tartaric acid: ethylene glycol: the sum of metal ions=1.5: 3.5: 1.0, and adjust the pH of the reaction system to 1 with ammonia. The reaction system was dehydrated in a constant temperature water bath at 50°C to obtain a sol, and the dehydration was continued to form a wet gel, and then the wet gel was heated and dried at 100°C for 10 hours to obtain a honeycomb xerogel. Finally, the dry gel was heat-treated at 400° C. for 10 hours in an air atmosphere, and then cooled to room temperature with the furnace to obtain an electrocatalyst with a perovskite crystal structure.
[0032] Preparation of air diffusion electrode: Mix acetylene black and pore-forming agent with alcohol evenly, add polytetrafluoroethylene emulsion, stir continuously to make it disperse evenly, ...
Embodiment 2
[0035] Preparation of electrocatalyst: La(CH 3 COO) 3 , Sr(CH 3 COO) 2 , Co(NO 3 ) 3 and Fe(NO 3 ) 3 , add deionized water to make a saturated aqueous solution, then add malic acid and glycerol in the above reaction system according to malic acid: glycerol: the sum of metal ions=2.5: 4.5: 1.0, adjust the pH of the reaction system with ammonia water to be 3 . The reaction system was dehydrated in a constant temperature water bath at 70°C to obtain a sol, and the dehydration was continued to form a wet gel, and then the wet gel was heated and dried at 150°C for 5 hours to obtain a honeycomb xerogel. Finally, the dry gel was heat-treated at 650° C. for 5 hours in an air atmosphere, and then cooled to room temperature with the furnace to obtain an electrocatalyst with a perovskite crystal structure.
[0036] Preparation of air diffusion electrode: Mix acetylene black and pore-forming agent with alcohol evenly, add polytetrafluoroethylene emulsion, stir continuously to make...
Embodiment 3
[0039] Preparation of electrocatalyst: weigh Ce(NO 3 ) 3 ·6H 2 O, Ba(NO 3 ) 2 , Cu(NO 3 ) 2 and Mn(CH 3 COO) 2 , add deionized water to make a saturated aqueous solution, then add citric acid and ethylene glycol in the above reaction system according to citric acid: ethylene glycol: metal ion sum=2.0: 3.5: 1.0, adjust the pH of the reaction system to be 4 with ammonia water . The reaction system was dehydrated in a constant temperature water bath at 80°C to obtain a sol, and the dehydration was continued to form a wet gel, and then the wet gel was heated and dried at 200°C for 1 hour to obtain a honeycomb xerogel. Finally, heat-treat the dry gel at 1000° C. for 0.5 hour in an air atmosphere, and then cool down to room temperature with the furnace to obtain an electrocatalyst with a perovskite crystal structure.
[0040] Preparation of air diffusion electrode: Mix acetylene black and pore-forming agent with alcohol evenly, add polytetrafluoroethylene emulsion, stir con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com