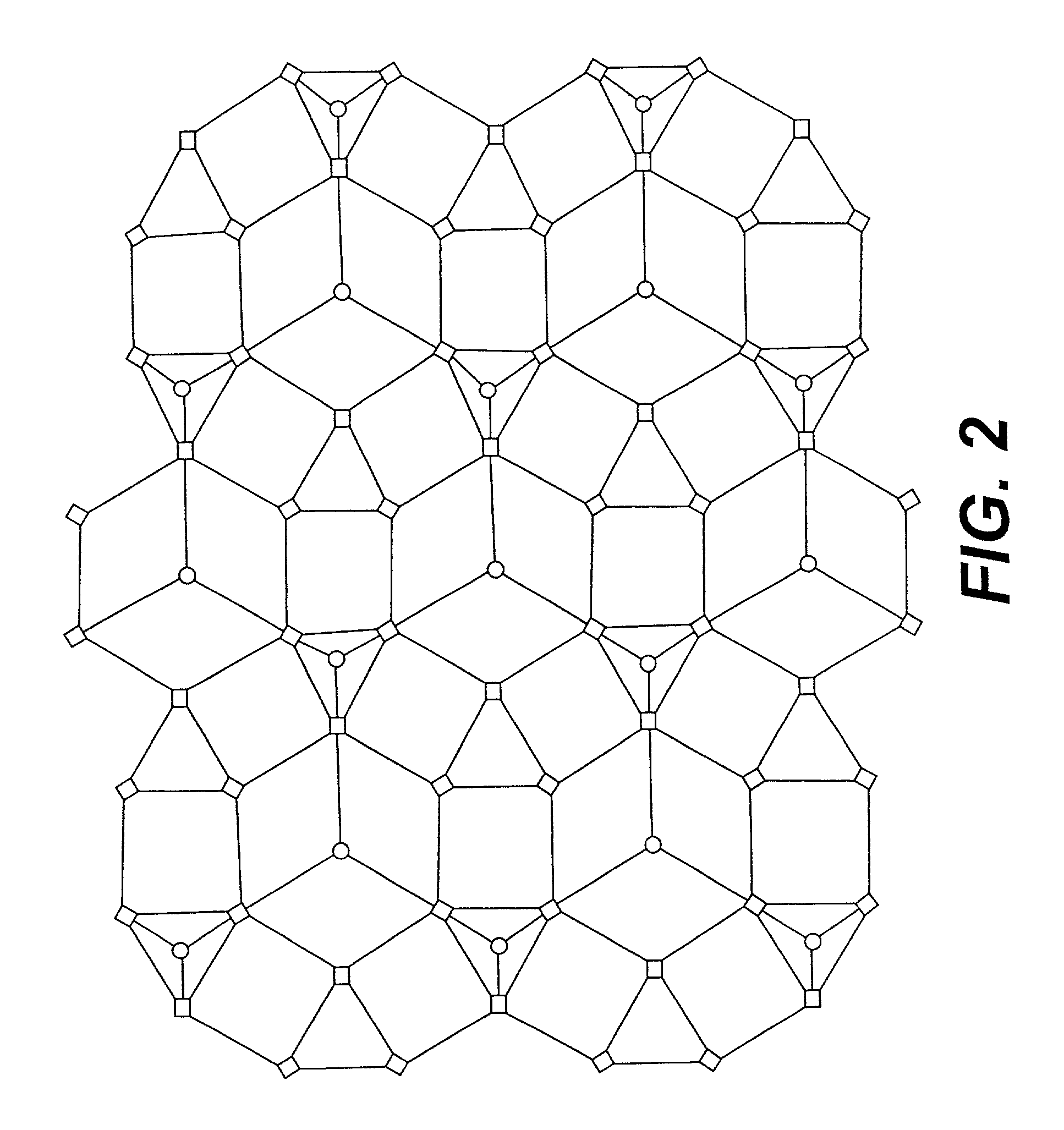
Three dimensionally periodic structural assemblies on nanometer and longer scales
a three-dimensional periodic structure and nanometer technology, applied in the field of three-dimensional periodic structure formation, can solve the problems of limited success in creating three-dimensional periodic structures, degrade the desired properties resulting from infiltrated materials, and achieve the effects of optimizing diffraction efficiency, enhancing diffraction efficiency of array of particle arrays, and maximizing diffraction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example
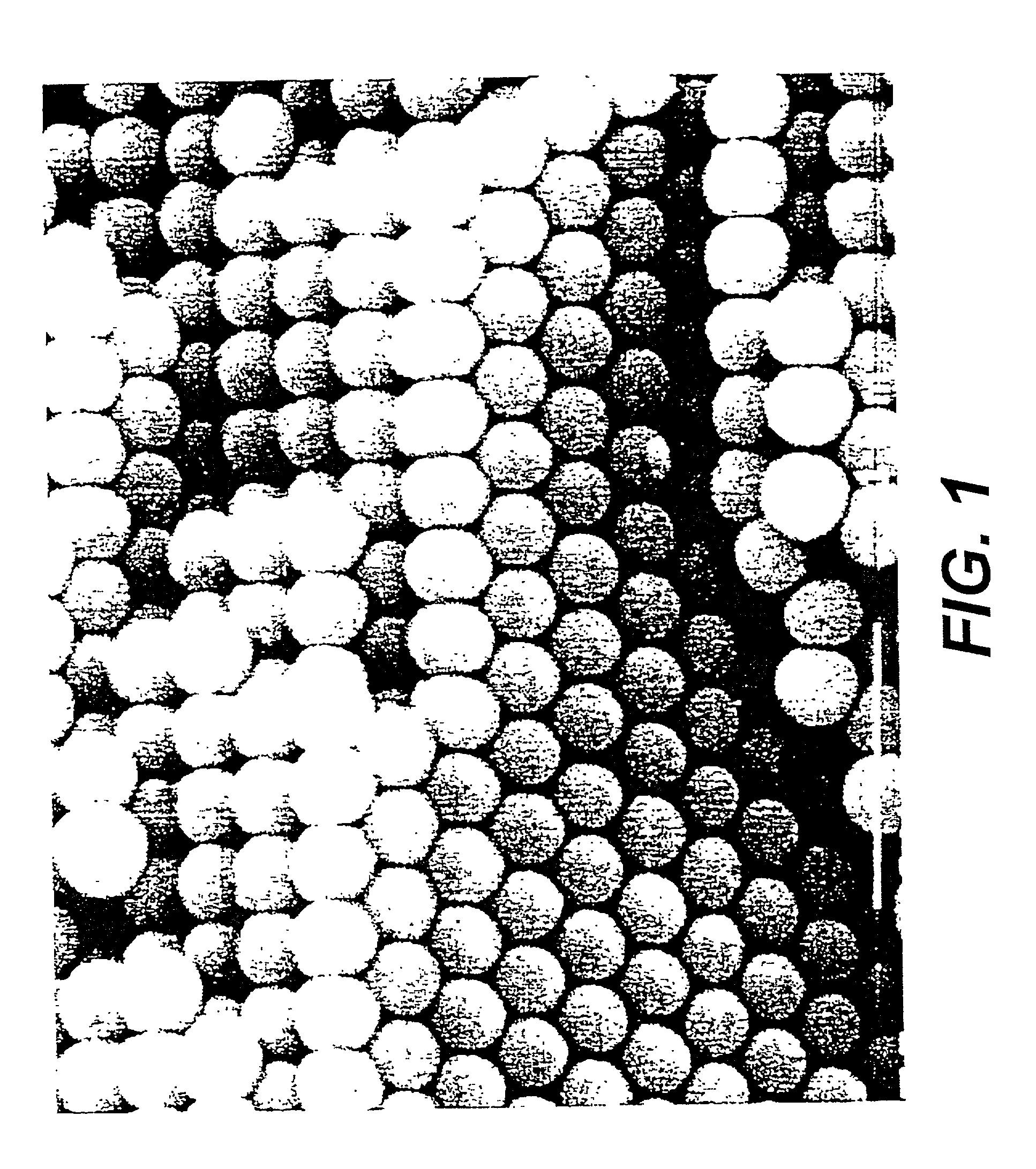
[0111] This example demonstrates the fabrication of a phenolic inverse opal by the templating of a sintered porous opal that has a periodic structure at optical wavelengths FurCarb.RTM. Resin (187 g, LP-520, which is a furfuryl-based phenolic resin available from Great Lakes Chemical Corporation, P.O. Box 2200, West Lafayette, IN 47906) was vigorously stirred for ten minutes at room temperature after adding four drops of hydrochloric acid (37.5 wt %, Fisher) as the catalyst for polymerization. A piece of sintered porous opal (FIG. 1) composed of 250 nm SiO.sub.2 spheres was placed into a small Teflon coated aluminum cup containing about 1.5 gm of the above resin containing HCl catalyst. After two days at room temperature, during which time the m resin infiltrated the opal, the resin was cured in an oven for three days at 80.degree. C., two days at 100.degree. C., and finally two days at 130.degree. C. The cured resin was black. After removing the cured resin around the resin-infiltr...
example 2
[0112] This example demonstrates the intense diffraction-based coloration of the polymer inverse opal and the switching of coloration. A piece of the phenolic inverse opal was prepared according to the previous example. It showed a brownish light-blue color. This inverse opal was broken into four pieces, which were placed in hexane, ethanol, acetone, and water, respectively. The sample in hexane showed a bright green color. The one in acetone gave red, green, and yellow opalescence depending on the orientation of the sample region with respect to the incident light. The one in ethanol offered a light yellow-green color. The one in water did not substantially change its color from that of the liquid-free inverse opal. These differences in coloration in the different liquids are attributed to the difference between the refractive indices of these liquids (which are 1.375, 1.359, 1.357, and 1.333 for hexane, ethanol, acetone, and water, respectively).
example 3
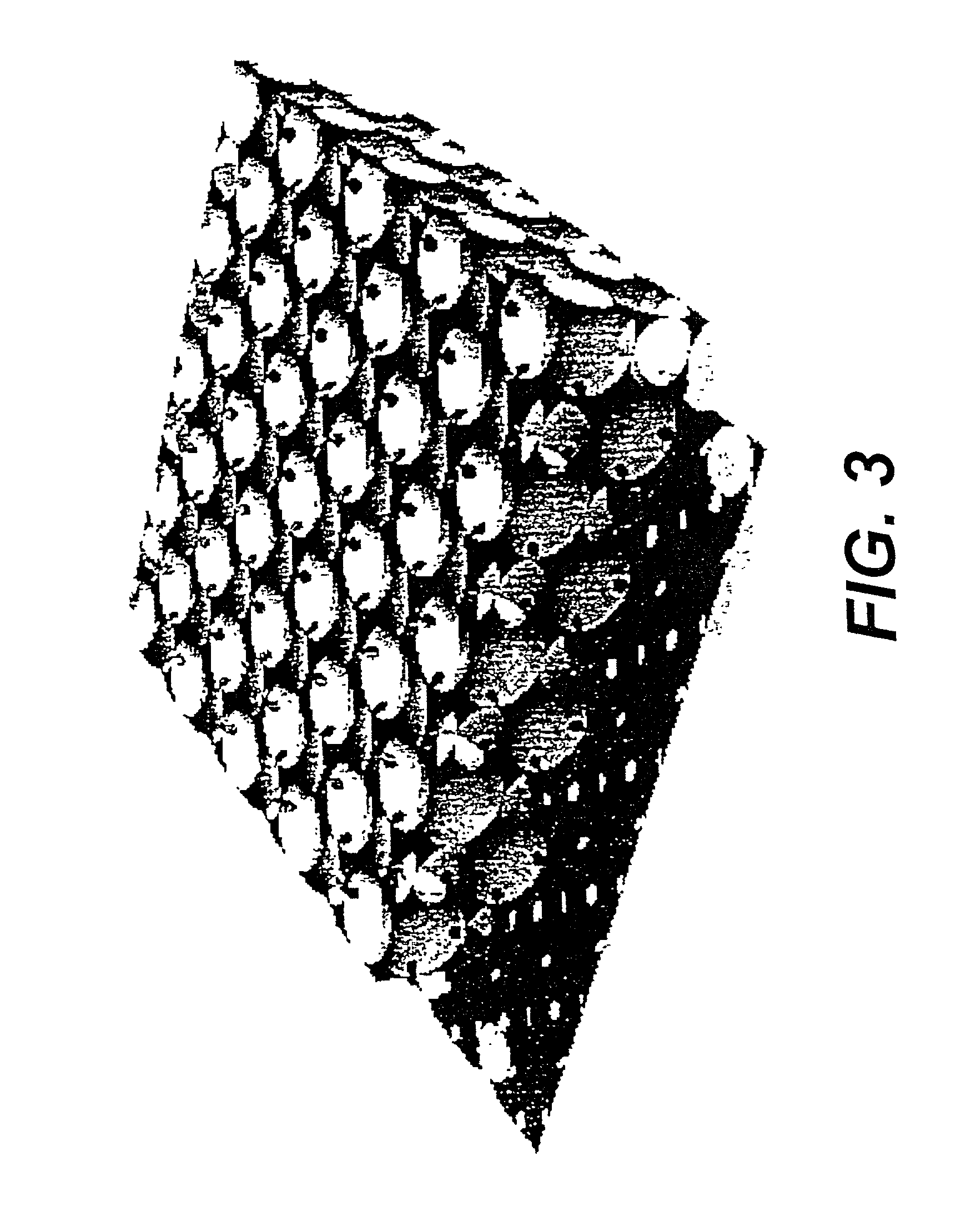
[0113] The samples comprising cured phenolic resin in SiO.sub.2 opal were prepared according to the procedure in Example 1. These samples were then embedded in the powder of the cured resin and carbonized under argon using the following thermal process. The sample temperature was increased from room temperature to 750.degree. C. in five hours, maintained at 750.degree. C. for three hours, and then cooled down to room temperature without temperature control. After the samples were treated with an oxygen-plasma for 5 minutes, they showed opalescence on a dark-black background. Then the samples were further treated with hydrofluoric acid for 2.5 hours, repeatedly washed with water, and dried over anhydrous CaSO.sub.4. This overall process, used for removing the silica spheres decreased the sample weight by about 87 to 90%, but did not cause a change in sample size and shape. This sample was opalescent and did not noticeable change coloration when immersed in liquids having various refr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameters | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com