Multi-use multimodal imaging chelates
a multi-modal imaging and chelate technology, applied in biochemistry apparatus and processes, organic active ingredients, organic chemistry, etc., can solve the problems of limited degree to which a complete brain resection can be carried out, difficulty in visually detecting differences between normal brain tissue and malignant tissue, and inability to over-emphasize the importance of imaging and detection techniques, etc., to achieve easy modification of spectral properties and biospecificity, improve brain cancer clinical outcomes, and facilitate macroscopic scal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
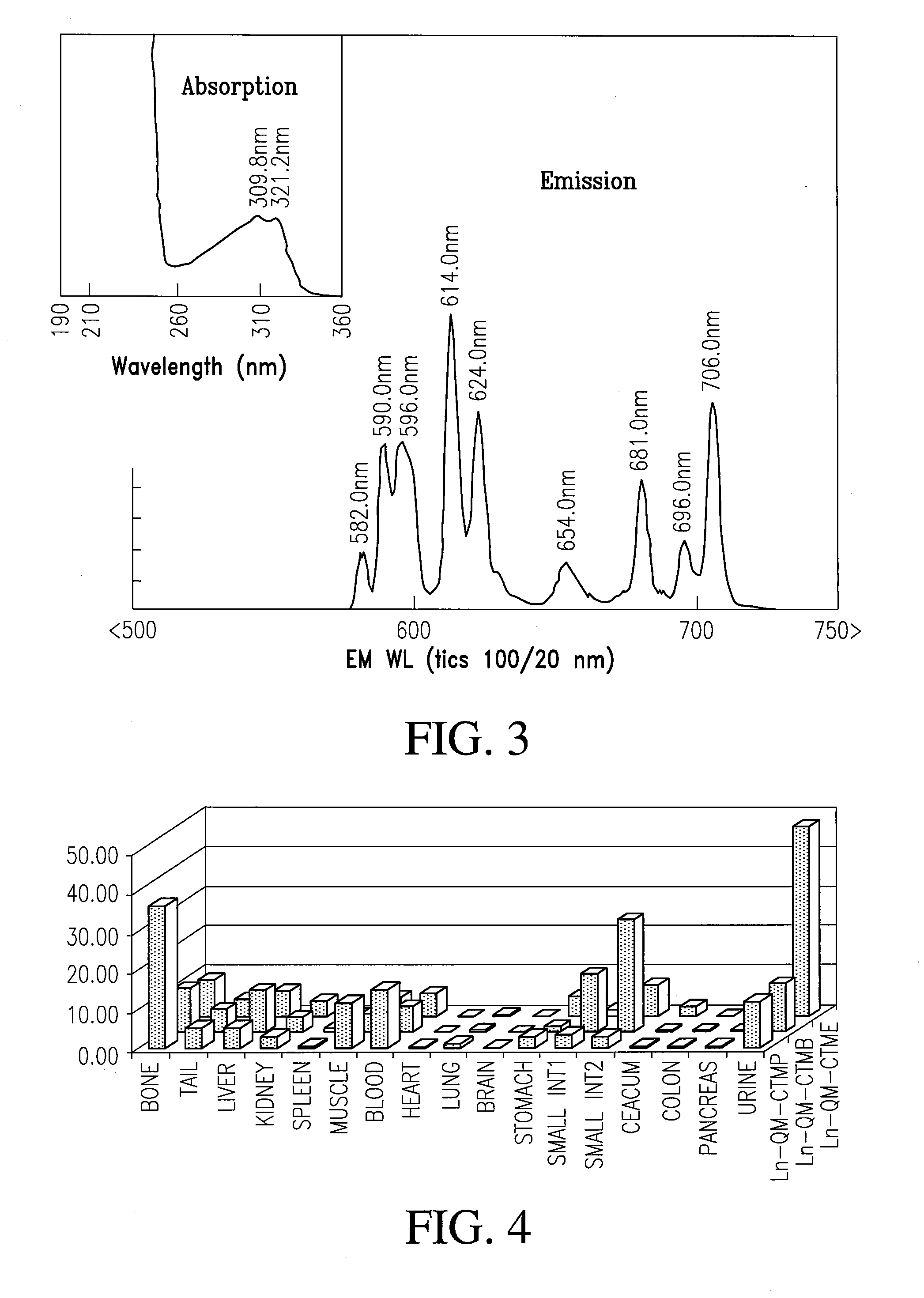
Image
Examples
example 1
Method of Making Cyclen-Based Lanthanide Chelates
[0059]Scheme I shows a method of preparing a first class of cyclen-based lanthanide chelates by attaching quinoline antennae to cyclen and then functionalizing the macrocycle.
[0060]First, to a stirring solution of cyclen (3.52 g, 0.0204 mol) in chloroform (525 mL) was added 2-(Chloromethyl)-6-fluoroquinoline 19 (2 g, 0.0102 mol). The reaction was then allowed to stir until completion as determined by TLC, concentrated and purified on silica using a gradient elution system starting with 50:1 CHCl3:MeOH; 150:4:1 CHCl3:MeOH:NH4OH; 100:4:1; 50:4:1; and finally with 20:4:1 to afford 2.54 g (75%) of a pale yellow oil that solidified on standing to an off-white solid. The resulting compound is N-(6-fluoro-2-quinolylmethyl)-1,4,7,10 tetraazacyclododecane 21.
Formation of N-(6-fluoro-2-quinolylmethyl)-N′,N″,N′″-tris(methylene phosphonic acid)-1,4,7,10 tetraazacyclododecane 25, wherein Y═F
[0061]To a stirring solution of the resulting N-(6-fluoro...
example 2
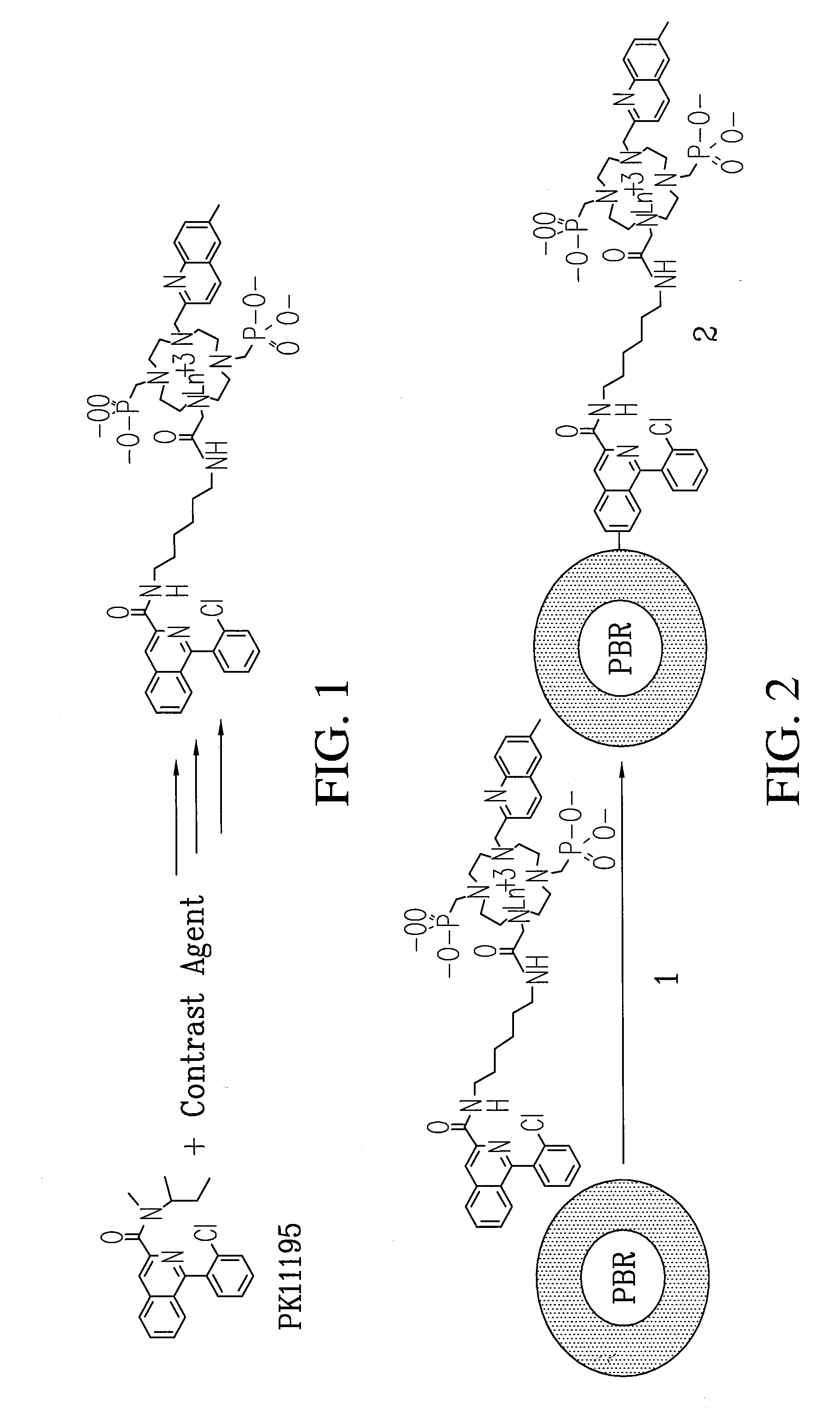
[0070]A tri-functional cyclen-based lanthanide chelate may be used to facilitate molecular imaging and allow site-specific delivery. This tri-functional chelate is conjugated, via standard linking chemistry to a carboxylate group, is light sensitized through the antenna and is a strong ligating complex through the phosphonate pendent arms and heterocycle. This is a facile and efficient synthesis that can be used to make numerous imaging agents. In addition, the resulting 6-substituted quinaldines have light stability and are very inexpensive to prepare.
Method of Making
[0071]The general synthesis used to accomplish this task is shown in Scheme II and provides a vehicle to make numerous molecular imaging agents for targeted delivery, all of them having unique and improved absorption properties.
[0072]As depicted in Scheme II, the synthesis of a carboxylate derivation of a cyclen-based lanthanide chelate is produced using the following method. First, 1,7-Bis(bezyloxycarbonyl)-1,4,7,10-t...
example 3
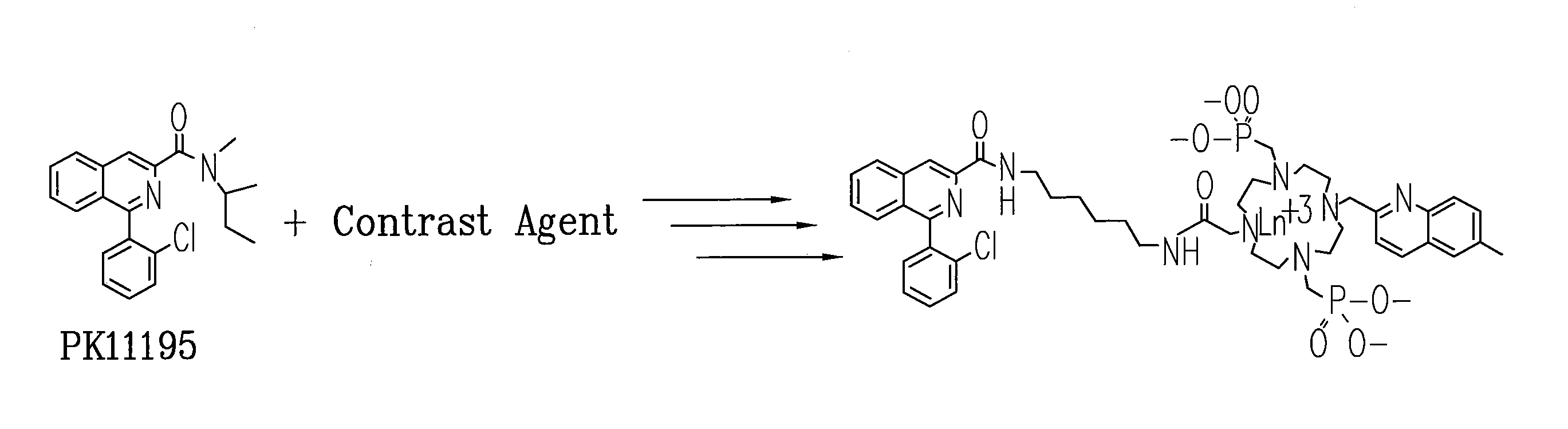
[0078]Subsequently, the cyclen-based lanthanide chelates obtained in EXAMPLE 2 can be conjugated to PK-11195 to give specificity of uptake and numerous signatures for co-registration diagnostic imaging and surgical guidance. Previous studies on PK-11195 and a closely related compound indicate that it can be conjugated, will retain it biological activity and can therefore be used as a molecular imaging agent to detect blastoglioma and study mitochondrial function.
Method of Making Conjugable PK-11195
[0079]An Ln-PK-11195 conjugated chelate (Eu-QM-CTMC-PK11195) can be prepared and will provide useful fluorescence and MR images. Scheme III illustrates a process for making a small quantity of high purity conjugable PK-11195.
A conjugable form of PK-11195 is formed by first producing 2-Chloro-N-(2-hydroxy-1-methyl-2-phenylethyl)benzamide 75 by the following process. To an ice-cooled mixture of Norephedrine 71 HCl in CH2Cl2 (120 mL, 5.2 g, 27.8 mmol) and 40 mL of 5% NaOH was added dropwise a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature sensitive | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com